Key Points
Disease summary:
Familial hypercholesterolemia (FH) is an autosomal dominant, monogenic disorder of lipoprotein metabolism characterized by strikingly elevated low-density lipoprotein-cholesterol (LDL-C), the presence of xanthomas, and premature atherosclerosis.
FH is most often caused by a defect in the gene that encodes for the apolipoprotein B or E (ApoB or E) (LDL) receptor (LDLR). Over 1000 different mutations of this receptor have been identified since it was first discovered by Goldstein and Brown in the late 1970s.
Impairment in, or in severe cases, a complete absence of function of, LDLR results in reduced clearance of LDL particles from the circulation into many cell types. Because over 60% of total body LDLR is in the liver, decreased clearance of LDL particles by this organ has a particularly potent effect on plasma LDL-C levels. Hypercholesterolemia is present from birth. LDL particles begin to be retained in arterial sites early on in life and their uptake by macrophages turn them into foam cells, the fundamental building block of an atherosclerotic plaque.
The FH homozygote frequency is estimated to be 1:1,000,000 worldwide compared with the heterozygote frequency of 1:500.
Differential diagnosis:
The differential diagnosis includes sitosterolemia, cerebrotendinous xanthomatosis, familial combined hyperlipidemia, polygenic hypercholesterolemia, familial defective ApoB-100, autosomal recessive hypercholesterolemia (ARH), and cholesterol 7 alpha-hydroxylase deficiency based on either clinical presentation (usually xanthoma) or laboratory testing (elevated LDL-C), or family history of elevated LDL or premature coronary artery disease (CAD).
Monogenic forms:
Most often caused by a mutation of one or both copies of the gene that encodes for the LDLR, located on chromosome 19p13.
Family history:
The prevalence of heterozygous FH is drastically higher in first-degree relatives of patients with the disorder estimated at one in two. Having a known case of FH in the family lowers the LDL cutoff needed to confirm the diagnosis (Table 15-1).
Environmental factors:
For patients with homozygous FH, risk is related to an extremely high level of LDL-C that is relatively insensitive to modifications by lifestyle factors. In contrast, for heterozygous FH, the phenotypic expression of the disease is related not only to the severity of the LDLR defect, but also to traditional dietary, genetic, behavioral, and cultural factors. For example, Hill et al. studied a large cohort of heterozygous FH patients and found that men who smoked or had low HDL levels had the greatest risk for developing CAD. This was in contrast to women in whom CAD was most associated with elevated triglycerides or the presence of hypertension.
Genome-wide associations:
Not surprisingly, the LDLR comes up in genome-wide association studies (GWAS) as associated with both LDL-C levels and risk of myocardial infarction. There are also a number of other genes that are significant in determining LDL-C levels that are not related to the LDLR, such as ApoB, proprotein convertase subtilisin/kexin type 9 (PCSK9), and sortillin. An important lesson from the GWAS on lipids, the largest incorporating up to 100,000 individuals of European descent, is that the same genes that cause Mendelian disorders also harbor common variants that result in small but significant changes in lipid levels. Much of the remaining inheritability may ultimately be attributable to these novel variants.
Pharmacogenomics:
Many believe that the response to treatment is likely a function of the particular mutation causing FH but few studies have been conducted to answer this question rigorously.
Diagnostic Criteria and Clinical Characteristics
Conventionally, the diagnosis of FH has been based on elevated total cholesterol levels in subjects belonging to families with high frequencies of premature CAD. Tendon xanthomas are thought to be nearly pathognomonic of FH, but are insensitive as a diagnostic marker.
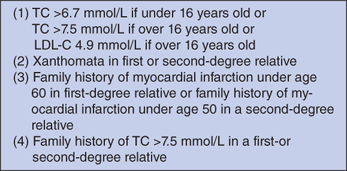
Three sets of diagnostic criteria utilizing population-based algorithms have been used extensively for clinical diagnosis: the Simon Broome Register Group in United Kingdom, the Make Early Diagnosis to Prevent Early Death program in United States, and the Dutch Lipid Clinic Network (Table 15-1 and Fig. 15-1).
| LDL-C Cut Point (MG/DL) | ||||
|---|---|---|---|---|
| Age (Years) | First- Degree Relative | Second- Degree Relative | Third- Degree Relative | General Population |
| > 20 | 155 | 165 | 170 | 200 |
| 20–29 | 170 | 180 | 185 | 220 |
| 30-39 | 190 | 200 | 210 | 240 |
| ≥ 40 | 205 | 215 | 225 | 260 |
Laboratory data should show an elevated total cholesterol level, a plasma LDL-C level above the age- and gender-related 95th percentile for the local population, a normal triglyceride level, and often a slightly low serum HDL-C. Secondary causes of hyperlipidemia such as thyroid disease and kidney disease should be excluded. Once there is high clinical suspicion, genetic testing for an LDL-receptor mutation known to cause FH is the only way to make an unequivocal diagnosis. After a mutation has been identified for a specific pedigree, genetic mapping can be used to identify individuals with FH. The large assortment of mutations known to cause FH makes screening the general population impractical.
The characteristic findings on physical examination are excessive cholesterol deposits which are either visible or palpable. The Achilles tendon is the most common site of xanthoma formation (tendon deposits), followed by the dorsum of the foot, extensor tendons of the hand, and the tibial tuberosity. Deposits in the eyelid (xanthelasma) or cornea (arcus cornealis) are common but not specific for FH.
Homozygous FH patients express few, if any functional LDLRs as a result of mutations to both alleles and have plasma LDL levels that can reach greater than 1000 mg/dL. Tendon xanthomas, cutaneous xanthoma (yellow-orange lesions on the skin), and CAD are regularly seen before the age of 25, and in some, as early as 4 years of age. Approximately 50% will also develop a characteristic supravalvular aortic stenosis due to atherosclerotic involvement of the aortic root.
Heterozygous FH patients have plasma LDL-C levels two- to fivefold higher than normal and without treatment, clinically significant CAD usually occurs at the mean age of 45 to 48 years in men and 55 to 58 in women. The prevalence of xanthoma increases with age, eventually occurring in 70% of heterozygous patients.
Screening and Counseling
The wide array of mutations and phenotypic variability seen in FH, as well as mutations in other genes, such as ApoB and PCSK9, which can cause clinical and laboratory presentations similar to FH, in conjugation with the lack of sophisticated diagnostic and public health resources, has made it difficult to develop a cost-effective, genetic screening program for the general population, or even those with premature CAD.
Patients deemed to have definite, possible, or probable FH based on the population-based algorithms described earlier should have family screening performed at a center with expertise in lipid risk management. Screening for this disease can begin as early as age 2 to 3 years if suspicion is high.
Cascade testing (screening relatives of FH probands) can be performed with the same clinically based criteria as described earlier. However, some data suggests that up to 25% of family members could be incorrectly classified on the basis of cholesterol testing alone.
The Dutch have the most experience with cascade testing using genetic testing. From data collected, this method would likely identify 60% to 80% of patients with definite FH, but much fewer when the diagnosis is only probable or possible. The main drawbacks are that many mutations in the LDLR may still be unknown, there are likely genes responsible for phenocopies of FH which are yet to be identified, and current techniques are relatively insensitive at screening the whole LDLR gene.
Although FH is monogenic and penetrance is almost complete, the phenotypic expression, in terms of onset of severity of atherosclerotic disease, varies considerably even among individuals who share the same genetic defect. As mentioned earlier, for heterozygotes in particular, the phenotype depends not only on the level of dysfunction of the LDLR, but also genetic, metabolic, and environmental factors.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree