|
Typical Clinical Features |
Microscopic Features |
Ancillary Investigations |
Calcifying fibrous tumor |
Children, young adults, M = F
Abdomen up to 15 cm
Can be multiple
Also in subcutaneous and deep soft tissue |
Circumscribed, unencapsulated tumor with hypocellular collagen containing scanty fibroblasts, inflammatory aggregates, and rounded calcifications |
Occasionally CD34+ or SMA+ |
Reactive nodular fibrous pseudotumor |
M > F, solitary or multiple, mesentery and surface of small or large bowel, circumscribed
History of abdominal surgery in some |
Nodules of mildly to moderately cellular stellate and spindle cells in fascicles in collagen, lymphoid aggregates |
SMA+, desmin±, CK ± |
Heterotopic mesenteric ossification |
Follows abdominal surgery or trauma, can present with intestinal obstruction |
Resembles ossifying fasciitis with nodular fasciitis-like background and foci of metaplastic bone formation |
SMA+, desmin−, h-caldesmon− |
Retroperitoneal fibrosis |
M > F, plaque at aortic bifurcation, ureters retract |
Collagen bands, mixed inflammation, vasculitis, few spindle cells |
SMA+, IgG4+ in plasma cells |
Sclerosing mesenteritis |
M > F, ill-defined mesenteric mass
Can be associated with other sclerosing fibroinflammatory diseases |
Fibrosis, inflammation, fat necrosis, foamy cells, sparse spindle cells |
SMA+, IgG4+ in plasma cells |
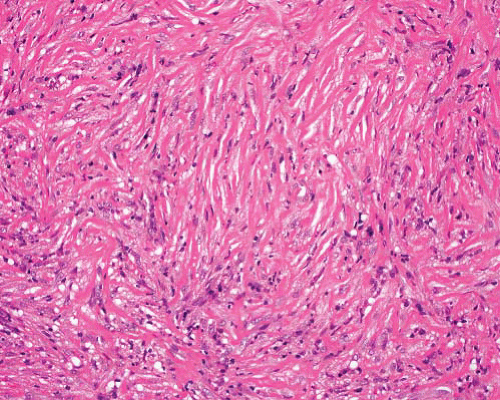
Fibromatosis—desmoid type |
Any age, deep, limbs, head and neck, body cavities
Association with familial adenomatous polyposis |
Parallel myofibroblasts evenly dispersed in collagen, slit-like and muscular-walled small vessels, and mast cells
Focally myxoid or with keloidal fibers |
SMA+, beta-catenin+ (nuclear) |
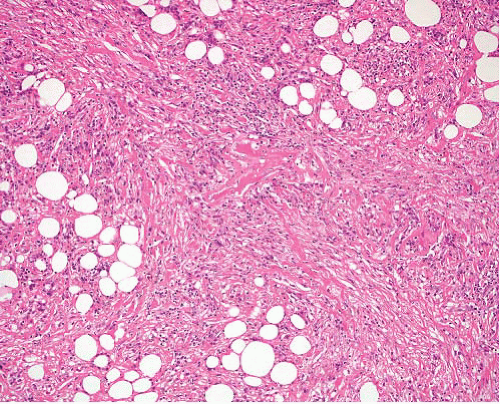
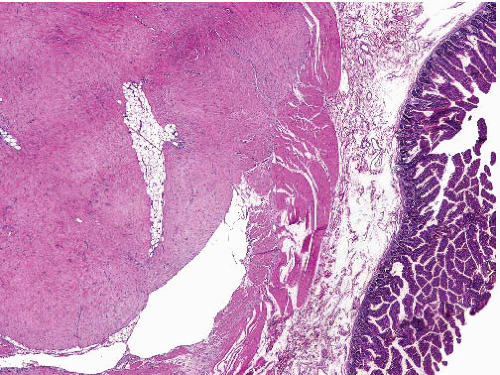
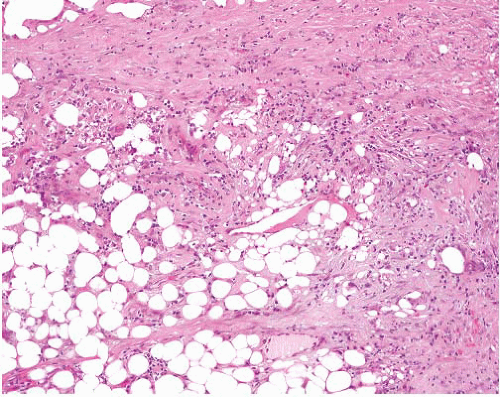
Sclerosing well-differentiated liposarcoma |
Older adults
Often multicentric |
Variable admixture of paucicellular sclerosis and adipose tissue, with scattered enlarged hyperchromatic nuclei mostly in fibrous areas
Rare lipoblasts |
CDK4+, MDM2+, P16+, CD34+, FISH shows amplification of CDK4, MDM2 |
Solitary fibrous tumor |
Mass in pelvis, abdomen, or retroperitoneum |
Circumscribed, not usually encapsulated, distinct cellular and fibrous areas, focal myxoid stroma, patternless short spindle cells
Hemangiopericytomatous pattern focally
Malignant variant has hypercellularity, mitoses >4 per 10 hpf, necrosis |
STAT6+, CD34+, CD99+, bcl-2+ |
Inflammatory myofibroblastic tumor |
Childhood or adult
Single mass or multicentric
Mesenteric, retroperitoneal, other sites |
Spindle cells in fascicles or myxoid fasciitis-like patterns
Occasional larger polygonal cells
Focal sclerosis without spindle cells
Marked inflammation, plasma cells prominent especially in areas of sclerosis |
SMA+, ALK+ (about 55%, especially childhood visceral tumors)
ALK gene rearrangements |
Leiomyosarcoma |
F > M
Retroperitoneum, bowel wall, or wall of vessel including inferior vena cava, renal vein |
Fascicles at right angles
Cells elongated with eosinophilic cytoplasm and nontapered nuclei
Paranuclear vacuoles
Myxoid change, fibrosis
Inflammatory variant has bland spindle cells, marked lymphocytic infiltrate, foamy macrophages, psammoma bodies |
SMA+, desmin+, h-caldesmon+, CD117− |
Gastrointestinal stromal tumor |
Related to wall of any part of alimentary tract (most commonly stomach, small intestine)
Also, in retroperitoneum, omentum
Metastatic potential varies with site, size, and mitotic index per 50 hpf |
Fascicles of long spindle cells, nuclei with blunt or tapered ends, paranuclear vacuoles
Organoid pattern, palisading
Focal or widespread epithelioid morphology common
Occasional clear cell, plasmacytoid, or rhabdoid change |
CD117+, DOG1+, CD34+, h-caldesmon +. SMA variable, desmin+ rarely, S100 protein+ rarely. CK sometimes+ after therapy. KIT or PDGFRA mutations, some SDH deficient |
Inflammatory fibroid polyp |
Submucosa and mucosa of esophagus, stomach, or intestine |
Bland spindle and stellate cells in inflamed stroma including eosinophils
Perivascular whorls |
CD34+, CD117−, h-caldesmon−. PDGFRA mutations |
Clear cell sarcoma-like tumor of gastrointestinal tract |
Occurs in stomach or small intestine |
Nested pattern, round nuclei with central nucleolus, clear or granular cytoplasm, sometimes spindling, osteoclast-like giant cells |
S100 protein+, HMB45 and melan-A usually negative, CD56, synaptophysin and NSE+ in some
t (2;22)(q33;q12) with EWSR1-CREB1 fusion, or t(12;22)(q13;q12) with EWSR1-ATF1 fusion |
Perivascular epithelioid cell tumor |
Falciform ligament, mesentery, retroperitoneum, uterus |
Nests of ovoid or spindled cells with clear or granular cytoplasm, delicate fibrous septa
Malignant variants often epithelioid |
SMA+, HMB45+, melan-A+, desmin+ in some TFE3+ in some, S100 protein+ rarely |
Cellular schwannoma |
F > M
Middle age
Paravertebral in retroperitoneum or pelvis, can erode bone
Also submucosal in stomach or intestine |
Thick capsule, subcapsular lymphoid aggregates
Fascicles of cells with eosinophilic cytoplasm, focal pleomorphism, occasional mitoses
Lacks Antoni A and B areas
Lymphocytes, clusters of foamy cells, thick-walled vessels, hemosiderin |
S100 protein diffusely+, CD117−
Some cases express CK |
Dedifferentiated liposarcoma |
Older adults, large retroperitoneal tumor, recurrences frequent |
Low-grade dedifferentiation: cellular fascicles with mild pleomorphism |
CDK4+, MDM2+, variable desmin, SMA, CD34 positivity |
|
|
High-grade dedifferentiation: pleomorphic undifferentiated sarcoma, or myofibrosarcoma-like
Heterologous osteochondroid or rhabdomyosarcomatous elements |
FISH shows amplification of CDK4, MDM2 |
Sarcomatoid mesothelioma |
Sheet-like mass involving peritoneal surface or omentum
History of asbestos exposure |
Fascicles of pleomorphic spindle cells, tapered nuclei, scanty cytoplasm, mitoses, necrosis
Desmoplastic or hyalinized stroma
Epithelioid component in some |
CK focal+, calretinin+, CD34- and bcl-2− |
Follicular dendritic cell sarcoma |
Omentum, gastrointestinal tract, liver, spleen, soft tissue |
Sheets, whorls, and fascicles of ovoid cells
Prominent nuclear membranes, speckled chromatin
Intimate admixture of lymphocytes
Rarely giant cells, pleomorphism, necrosis |
CD21/35+, CD23+, S100 protein+, EMA+, D2-40+, fascin+, clusterin+, desmoplakin+, CD45− |
Synovial sarcoma, monophasic |
Very rare in abdomen
Mass in retroperitoneum or pelvis |
Sheets of uniform short spindle cells with minimal cytoplasm, focal pericytomatous pattern
Poorly differentiated synovial sarcoma is a small round cell tumor. |
CK, EMA, CD99, S100 protein+ focally, bcl-2+, TLE1+, CD34−, CD117−. t(X;18)(p11;q11), SSX-SS18 fusion gene |
Endometrial stromal sarcoma |
Can arise in a focus of endometriosis or present as metastasis in abdomen or elsewhere |
Short closely packed spindle cells, variable focal myoid differentiation
Thick-walled vessels |
CD10+, SMA+, occasional des+, CK+
Nuclear beta-catenin+ in 50%
ER, PgR+
JAZF1-SUZ12, JAZF1-PHF1, EPC1-
PHF1, or YWHAENUTM2A/B fusion genes |
Sclerosing lymphoma |
Adults, associated lymphadenopathy |
Cords and nests of atypical lymphoid cells with fibrous stroma
Absence of prominent spindle cell component |
Lymphoid markers, often large B-cell lymphoma |
Ig, immunoglobulin. |