Fig. 2.1
Phase transition temperatures of cholesterolpelargonate
The temperature of the transition solid–liquid crystal is called the melting temperature T m . The temperature of the transition liquid crystal–isotropic liquid is called the elucidation temperature T e . The term results from the fact that many crystals in the mesophase are turbid liquids scattering light considerably. The scattering disappears and the melt becomes transparent in transition to the isotropic liquid.
Liotropic liquid crystals are the substances which form the liquid-crystal phase when dissolving them in water or other solvents. As a rule, the phase appears within a strictly certain range of the concentration of the substance being dissolved in the solvent. Potassium n-alconate soap is a typical example of liotropic liquid crystals. Nowadays liotropic liquid crystals are very attractive for researchers. This is due to the fact that liotropic liquid-crystal phases are typical for more materials than thermotropic ones and to a great role of liotropic liquid crystals in biology. Note that myosin being an albumen contained in the contracting substance of the muscular tissue, desoxyribose nucleic acid playing a leading role in the transfer of heritable data, many polypeptides, ferments and other substances of biological origin are capable of forming the liotropic liquid-crystal phase [2, 3]. It is also found that liquid crystals are of great importance in metabolic processes in living organisms.
Like solid crystals, liquid crystals show the anisotropy of properties which is always closely related to the anisotropy of the matter structure [4]. Apparently, proceeding from this fact German chemist Vorlender supposed that liquid crystals were formed mainly from the organic compounds whose molecules had an elongated cigar-like shape, i.e. shown the anisotropy of the structure. His predictions had come true. It should be noted that earlier Leman assumed the structure of molecules of liquid-crystal substances to be anisotropic.
It is found that the substances with flat disc–shaped molecules also form the liquid-crystal phase. Such substances are called discotic liquid crystals or discotics.
Elongated cigar-shaped molecules of the liquid crystal are always in thermal motion. The direction of their major axes continuously changes relatively to a certain direction. To mark the direction of predominant orientation the unit vector n is used which is called “director ” . The director characterizes phenomenologically the long-range order of molecules.
Because of the thermodynamic least free energy principle the elongated or disc-shaped structure of liquid crystal molecules a certain arrangement of the molecules appears in the mesophase. The pattern of the arrangement is governed by both the structure of the liquid crystal molecules and thermodynamic conditions.
2.2 Classification and Structure of Liquid Crystals
The classification of liquid crystals was proposed by French physicist Fridel in the beginning of the 19th century and is based on molecular arrangement. Like Reinitzer and Leman, he examined microscopically thin layers of liquid crystals formed on a table glass in melting. He found that irrespective of the chemical structure of the compounds liquid crystals formed a limited number of kinds of optical pictures. Fridel related the kind of the optical picture observed with the arrangement of molecules in the samples and proposed the classification of liquid crystals on this basis.
He divided liquid crystals into smectic (from the Greek word “smegma” meaning soap) and nematic (from the Greek word “nema” meaning thread) crystals. Nematic liquid crystals are divided into properly nematic and cholesteric crystals. Two following facts resulted in such division. The first fact was that no mesogens were found passing consecutively the nematic and cholesteric mesophases . The second fact was that it was possible to transform a properly nematic liquid crystal into a cholesteric one and vice versa by external effects such as mechanical deformation, electric and magnetic fields.
In smectic liquid crystals molecules are arranged so that their major axes are parallel and their centers of mass lie in one plane. Major axes of the smectic molecules or the director n form an angle β with the normal to this plane (Fig. 2.2).
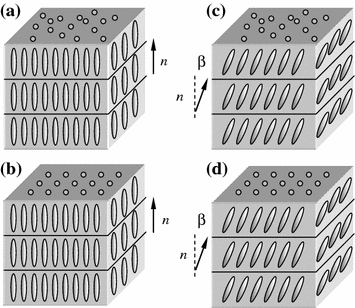

Fig. 2.2
Structure diagram of smectic liquid crystals with non-structured (a) and structured (b) layers
The location of the centers of mass of the molecules results in the formation of smectic layers. It is apparently that the thickness of the layers depends on the molecule length and the angle between the director n and the location plane of the centers of mass.
By the pattern of arrangement in the layers smectic liquid crystals are divided into two groups: with structured and non–structured layers . In smectic crystals with structured layers the centers of mass of the molecules form a two-dimensional lattice in the layer. The director can be oriented relatively to the layer both normally (Fig. 2.2a, b) and at an angle (Fig. 2.2c, d). Smectic crystals with structured layers are ordered better than crystals with non-structured layers. In smectic crystals of both groups the mutual slip of layers can occur and in most cases the rotation of molecules about their major axes is possible.
In non-structured layers molecules of the smectic crystal are distributed chaotically (Fig. 2.2a, c). This group consists of several subgroups of smectic crystals which differ in the angle of director orientation relatively to the normal to the layer.
Smectic crystals for which β = 0 (Fig. 2.2a) belong to the first subgroup. Therefore, in these crystals major axes of the molecules are perpendicular to smectic layers. Such crystals are commonly called smectics A. They are most widespread among smectic crystals.
The second subgroup consists of non-structured smectic crystals for which β ≠ 0. They are called smectics C (Fig. 2.2c). Among them are smectics C with a small (β < 30°) and great (β ≈ 45°) angle between the director and the normal to the layer. With elevating temperature smectics C with the small slope angle transform into smectics A. However, the smectics C are known for which the angle β depends on the temperature within a certain temperature range and can decrease down to zero while other smectics with the small slope angle show no temperature dependence of the slope angle β.
Smectics C with the great angle β do not transform into smectics A and their angle β is independent of the temperature.
If molecules of the smectic substance are chiral, i.e. twisted about their major axes, the twisted smectic mesophase C * is formed (Fig. 2.3).
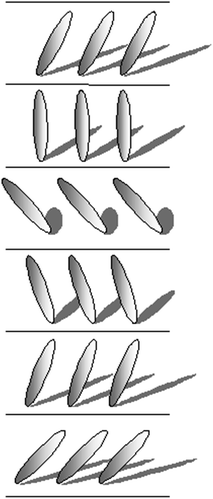

Fig. 2.3
Smectic C * mesophase
In such smectics the director rotates along the taper generatrix when passing from one layer to another.
In smectics B centers of mass of molecules in layers are located at points of the hexagonal lattice and the director is perpendicular to layers (Fig. 2.4).
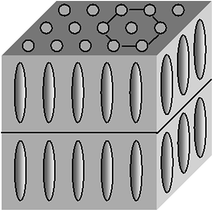

Fig. 2.4
Smectic B mesophase
A similar location of centers of mass is typical for smectics H but the director n is inclined to layers (Fig. 2.5).
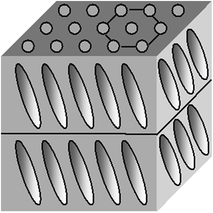

Fig. 2.5
Smectic H mesophase
If the smectic H forms a compound with chiral molecules the quasi-hexagonal arrangement remains but when passing from layer to layer the director rotates along the taper generatrix as in case of the smectic C * . This smectic is called smectic H * .
The quasi-hexagonal arrangement of centers of mass of molecules in layers is also typical for smectics E but unlike other smectics with structured layers the rotation of the molecules about their major axes is hampered. As the director is perpendicular to the layers, rhombic arrangement occurs (Fig. 2.6).
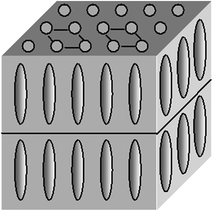

Fig. 2.6
Smectic E mesophase
In smectics G the arrangement of centers of mass of molecules and the hampering of rotation about major axes are the same as in smectics E but the director is inclined to the layer plane (Fig. 2.7).
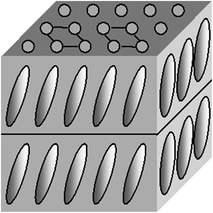

Fig. 2.7
Smectic G mesophase
It consists of structure units containing many molecules; they are called micelles (from the Greek word mica meaning grain). In nematic liquid crystals molecules are parallel to each other and their centers of mass are arranged chaotically unlike smectic crystals (Fig. 2.9).
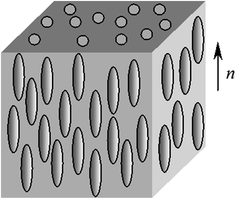

Fig. 2.9
Structure diagram of nematic liquid crystal
In this case layers typical for smectics are not formed and long-range order exists only in respect to the orientation of major axes of the nematic crystal.
Cholesteric liquid crystals are formed by cholesterol derivatives (esters), yet they may contain substances of other classes.
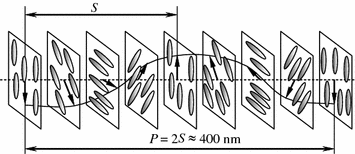
Cholesteric liquid crystals typically consist of molecular layers; each of them demonstrates the molecular arrangement specific for nematics when centers of mass lie in the molecular plane chaotically. The director also lies in the layer plane [5]. The thickness of the molecular layer called also a quasi-nematic layer [6] is 0.5–0.6 nm. In transition from one molecular layer to another the director rotates by a small angle relatively to the director of the underlying layer. This angular shift accumulates in the sequence of the layers and, generally, ends of the molecules or the director end move along a spiral (Fig. 2.10). Apparently, the lead P of the spiral is
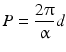
where α is the shift (twist) angle of the director in transition from layer to layer; d is the thickness of the molecular layer of the cholesteric crystal.
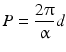
(2.1)

Fig. 2.10
Structure diagram of cholesteric liquid crystal
According to the results reported in [7], the angular shift of the director in transition from layer to layer in cholesteric crystals is 15 angular minutes on the average. Other authors give the value of about 0.5° [8]. Further thorough studies have shown that the twist angle α depends on both the temperature and the molecular structure of the cholesteric liquid crystal as well as some other factors [9–11].
Taking α = 0.5° and d = 0.6 nm we obtain that the lead of the cholesteric spiral is 400 nm.
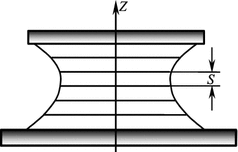
It is apparently that in the molecular layers corresponding to the director twist angles of 0°, π, and 2π the major axes of liquid crystal molecules have the same orientation (Fig. 2.10) that makes the layers indistinguishable. Then the distance S = P/2 is the period of the specific lattice of the cholesteric liquid crystal. It is easy to note that in cholesteric crystals the molecular orientation is repeated at a distance equal to the period S. However, the molecules can move freely and exchange their positions in each molecular layer but their spiral arrangement with the period S remains unchanged.
Macrolayers consisting of many molecular or quasi–nematic layers in which the spiral arrangement is retained along the certain direction at a distance of one or more lattice periods are called monocrystal layers of cholesteric liquid crystals (Fig. 2.11). Such layers can be formed between bearing surfaces.