An understanding of the discipline of neurology is critical to the practicing speech-language pathologist (SLP) because the central and peripheral nervous systems control structures that produce and modify sound into speech, and language itself is organized and constructed solely within the brain.
This chapter provides a framework for understanding the neurologic approach to problems the SLP encounters; the framework is based in anatomical (Where is the lesion?) and functional (What is the lesion?) constructs. An illustrative case: Your grandmother telephones you, and, because you are an SLP, tells you that her neighbor has begun to “talk funny.” You think a moment; this could mean anything. You ask, “Has the usual content of her conversation changed or become inappropriately jocular or puerile? Is she now speaking in jargon or neologisms? Has her speech become softer or monotone? Is it more hoarse or slurred? Is she saying funny things or are the words coming out funny?” Asking these questions is an attempt to ascertain where the problem is coming from (anatomical localization) and what is causing it (functional substrate). Answers to these questions allow you to establish if Grandma’s neighbor has a disorder of speech and language (e.g., dysarthria, dysphonia, aphasia), a thought problem (e.g., confabulation), or a combination of both.
17.2 Neuroanatomy
The nervous system traditionally is divided into the central nervous system (CNS) and peripheral nervous system (PNS) ( ▶ Table 17.1, ▶ Fig. 17.1); the autonomic nervous system (ANS) or visceral system is considered a component of the PNS.
System | Structures |
Central | Cerebral hemispheres, subcortical white matter, basal ganglia, thalami, pons, medulla, cranial nerve nuclei, spinal cord |
Peripheral | Peripheral nerves, neuromuscular junctions, muscles, sympathetic and parasympathetic nervous systems |
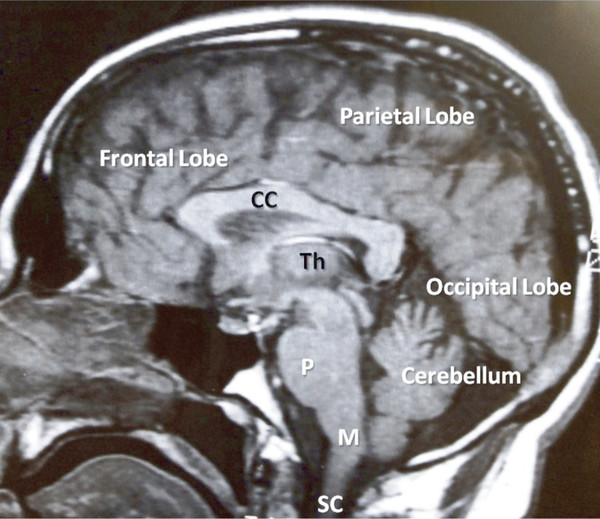
Fig. 17.1 The central nervous system. Sagittal magnetic resonance image showing major components of the central nervous system. Th, thalamus; CC, corpus callosum; P, pons; M, medulla; SC, spinal cord.
17.2.1 Higher Cortical Systems
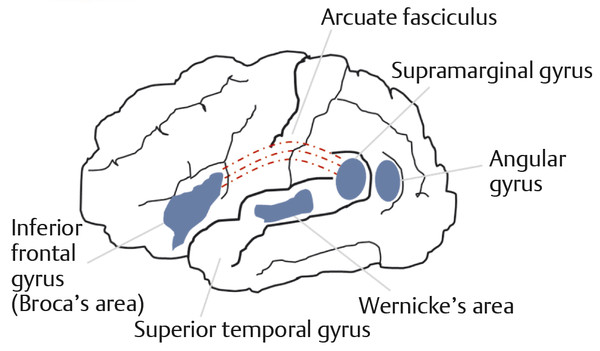
CNS structures that are involved in speech and language are distributed throughout the brain hemispheres. Each hemisphere classically is divided into lobes ( ▶ Fig. 17.1). The frontal lobe is involved in motor control; the parietal lobe receives sensory input from external (e.g., touch, proprioception) and internal stimuli; the occipital lobe is the primary structure for vision; the temporal lobe contains the center for hearing. The dominant hemisphere (most commonly the left) contains centers for generation of speech (Broca’s area) and interpretation of spoken and written language (Wernicke’s area) ( ▶ Fig. 17.2). The nondominant hemisphere is involved with the character or ‘emotionality’ of spoken language. Complex neural networks connect the brain structures involved in recognition, generation, interpretation, emotionality, and rhythmicity of language. All are influenced by input from systems involved in alertness, attention, memory, and behavior. Damage or dysfunction in the higher cortical systems can cause aphasia, language apraxia, or dysprosody. (Definitions of these terms are covered elsewhere in this book.)
Fig. 17.2 Diagrammatic representation of major language centers and their connections in the dominant hemisphere.
17.2.2 Motor Control
Motor Unit
Components of speech are organized hierarchically. Tissues that produce sound and modify speech are either largely muscle (e.g., the tongue) or soft tissues that change their shape through the action of attached or adjacent muscle(s). These skeletal muscles are innervated by lower motor neurons (LMNs), which have their cell bodies in the caudal (distal) part of the brainstem, specifically the lower pons and medulla. The neuromuscular junction (NMJ) functionally connects LMNs with the muscle, and together they form the motor unit. Damage or dysfunction of the motor unit leads to variants of dysarthria, which is inevitably flaccid.
Pyramidal System
The motor unit is under the control of several descending pathways that originate in the cerebrum or brainstem and are known collectively as the upper motor neurons (UMNs). UMNs project to the spinal (corticospinal) or cranial nerve (corticobulbar) centers where the LMNs originate. Corticospinal tracts descend through the pyramids in the medulla, hence the designation pyramidal tracts. Corticobulbar tracts provide the voluntary control over the cranial nerves that produce speech.
A lesion of the corticospinal tract causes weakness and a loss or reduction of contralateral voluntary movements in the extremities. On the other hand, unilateral lesions of the corticobulbar tract have less of an effect because most cranial nerves are controlled by both sets of corticobulbar tracts. A notable exception is the lower part of the face, which receives primarily contralateral (corticobulbar) input. Corticobulbar tract lesions have a marked effect on speech, manifesting as spastic dysarthria.
Extrapyramidal System
Descending pathways—other than the pyramidal tracts—that are collectively called the extrapyramidal system (e.g., the rubrospinal and reticulospinal), are primarily concerned with posture and tone of the upper and lower extremities, and their roles in the production of speech are less well understood. Also, other areas of the brain (e.g., basal ganglia and cerebellum) are involved with motor control and do so by way of complex feedback loops involving cortical and subcortical structures. The pyramidal and extrapyramidal motor systems are viewed as the “voluntary” and “involuntary” systems, respectively.
For example, consider a man standing ready to catch a baseball that is thrown to the right of him. When he reaches to the right to catch it, only the abduction and extension of the arm and the opening of the fingers are “voluntary.” The unconscious extension of the left arm and the requisite changes of the trunk musculature are “involuntary.” These changes have been largely planned and executed by the basal ganglia using real-time sensory information to maintain balance and utilizing prelearned motor programs to support the rest of the body in a stable posture while catching the ball. The cerebellum primarily smoothes and corrects the voluntary actions of the opposing muscles (i.e., agonist and antagonist) involved in the movements of the right arm as it is directed to and acquires the target.
17.2.3 Sensory Control
Primary somatosensory (bodily senses) modalities include (1) discriminative touch, which is required for recognition of size, shape, and texture of objects; (2) proprioception, which gives us the sense of static and dynamic position of body parts; (3) nociception, which signals pain and itch; and (4) temperature (warmth and cold). Discriminative touch and proprioception afferents are carried in the dorsal spinal column onto the thalamus, whereas nociceptive and temperature fibers run in the anterolateral spinal system on their way to the thalamus.
Primary sensory information ascends from the periphery through the spinal cord and brainstem to the thalamus in several tracts that differ in the type of information encoded. Thalamocortical fibers relay the information predominantly to the parietal cortex.
17.3 The Neurologic Evaluation
Components of the bedside neurologic evaluation include a history of the patient’s complaints, eliciting past medical, surgical, medication, social, and family histories, and a neurologic examination. The history of present illness (HPI) focuses on the patient’s current symptoms. A systematic approach to obtaining an HPI is critical and involves eliciting type, onset, temporal profile, severity, and duration of the presenting complaints. Relieving, aggravating, and associated factors equally are elicited. A useful mnemonic to remember when obtaining the history of a speech or language problem is P-Q-R-S-T, which stands for:
Precipitating (aggravating) factors and Previous occurrences
Quality (type) of symptom(s)
Relieving factors and Related (associated) symptoms
Severity (symptom severity; disability)
Temporal profile (onset, duration, frequency, acute vs. chronic, persistent vs. intermittent)
The neurologic examination is a structured approach to evaluating the nervous system function of the patient, and includes assessments of the mental status, cranial nerves, motor function, sensory function, reflexes, coordination, gait, and stance.
17.3.1 Mental Status and Higher Cortical Functions
The mental status examination is usually the first neurologic test, even if it is done informally. Typically, the following functions are screened: arousal, attention, memory, affect, speech and language, phonation, and drawing.
17.3.2 Cranial Nerve Testing
Olfactory Nerve (CN I)
The first-order terminals involved in perception of odors are contained in the olfactory nerves. Patients are asked whether they are able to perceive odors through one nostril while the other is occluded. Generally, small vials containing volatile agents (e.g., oil of wintergreen or camphor) are used. The inability to detect odors is termed anosmia.
Optic Nerve (CN II)
Although the rods and cones in the retina are the sense organs that convert light to electrical impulses, the fibers of the optic nerve are extensions of the first-order neurons of sight. The fibers of the optic nerve begin in the retina, exit the globe, and travel posteriorly to the optic chiasm, where a partial decussation or crossing occurs. The fibers continue and end primarily in what is known as the lateral geniculate nucleus of the thalamus. A small percentage of optic nerve fibers end in the midbrain as afferents of the pupillary light reflex.
CN II is tested by checking visual acuity with an eye chart as well as visual field testing. In the clinic, visual fields are usually tested by asking the patient to count or detect the movement of fingers or small objects in the visual fields. This is done with the contralateral eye covered and with the patient fixating on the examiner’s eye. The examination of the optic nerve also includes a funduscopic examination with an ophthalmoscope. Funduscopy allows direct examination of the retina, optic nerve head, retinal nerve layers, and the retinal vasculature.
Oculomotor, Trochlear, and Abducens Nerves (CNs III, IV, and VI)
The oculomotor, trochlear, and abducens nerves (CNs III, IV, and VI) provide the lower motor neuron control of eye movements. In addition, the third cranial nerve innervates the levator (elevator) of the eyelid, the constrictor muscle of the pupil, and the ciliary muscle that controls accommodation. CNs III, IV and VI functions are tested clinically by having the patient visually follow an object into the cardinal positions of gaze.
Trigeminal Nerve (CN V)
The fifth cranial nerve has both motor and sensory components. The motor component of the trigeminal nerve provides for jaw movement. Unilateral lesions are usually well tolerated, but severe bilateral lesions leave the mouth open and unable to be closed.
The sensory portion of CN V supplies sensation to the face as well as the buccal and nasal mucosa. The cells arise largely in the Gasserian ganglion and, like all sensory nerves, send processes distally to innervate the periphery and centrally to nuclei within the CNS. The fibers that course peripherally do so within the three divisions of the trigeminal nerve ( ▶ Fig. 17.3): the ophthalmic (V1), maxillary (V2), and mandibular (V3) branches. Centrally destined processes of the trigeminal sensory neurons enter the lateral pons and course rostrally or caudally in the brainstem depending on the type of sensory information they carry. Those concerned with pain and temperature descend into the medulla and upper cervical spinal cord as the descending trigeminal tract. Those carrying tactile (touch) and proprioceptive (position sense) information ascend to the main sensory nucleus. The motor fibers of CN V leave the pons and innervate the muscles of mastication (the temporalis, masseter, and pterygoids) via the mandibular branch.
Fig. 17.3 The trigeminal nerve (CN V). Diagrammatic illustration of the three peripheral branches of the trigeminal nerve.
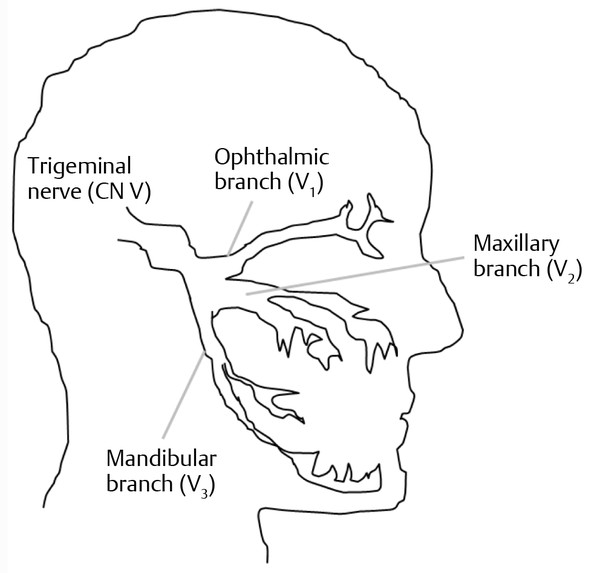
The maxillary and mandibular branches of CN V are of particular importance to the SLP since structures they innervate or control are involved in pronunciation, voice intonation, and swallowing. The maxillary branch carries sensation from the maxilla, maxillary teeth, the mucous membranes of the upper mouth, the anterior palate, nose and nasopharynx, the inferior portion of the internal auditory meatus, and the midface. The mandibular branch conducts sensory information from the skin of the cheek, the lower teeth and jaw, and the mucosal linings of the uvula, posterior hard palate, and nasopharynx. The mandibular branch contains the proprioceptive and other sensory information from the skin on the mandible, ipsilateral side of the tongue, and the buccal surface of the cheek.
The ophthalmic division can be tested with the corneal and nasal tickle reflexes. A wisp of cotton gently stroked across the junction of the sclera and cornea should elicit bilateral blinking. Similarly, a wisp of cotton introduced very gently into one of the patient’s nostrils should result in wrinkling of the nose. In both tests, the two sides are compared. The bulk of the masseter and temporalis muscles are tested by palpation of the muscles while patients clench their teeth. Once bulk has been ascertained, the strength of the muscles is tested by an attempt to open the patient’s jaw with downward pressure on the chin. The pterygoids provide for lateral movement of the jaw. The jaw will deviate to the side of the weak pterygoid and will be more easily pushed in that direction. Inside the mouth, weakness of the tensor veli palatini may manifest as tilting of the uvula away from the weak side. The jaw jerk reflex tests afferent and efferent function of the mandibular division of CN V.
Facial Nerve (CN VII)
The seventh cranial nerve also has motor and sensory components. It provides LMN innervation to the muscles of facial expression and the stapedius muscle, which is involved with stabilization of the stapes vibration in order to dampen external noise. The special sensory component of CN VII innervates the taste buds on the anterior two-thirds of the tongue. Parasympathetic fibers (components of the autonomic nervous system) within the facial nerve innervate the lacrimal, submandibular, and sublingual salivary glands.
Facial muscles are tested first by inspection of the face for symmetry and then by examining individual muscles for strength. Patients with weak facial muscles generally have fewer facial lines and wrinkles. The face looks inordinately placid. With unilateral weakness, the palpebral fissure will be wider on the weak side. When the orbicularis oculi are contracted maximally, the patient should be able to “bury” the origins of the eyelashes. Additionally, a good deal of resistance to forced eye opening should be apparent. The nasolabial folds should be roughly symmetrical; the weak side will appear flattened. With unilateral weakness, the corner of the mouth will be seen to sag. When smiling, the corners of the mouth should elevate. With bifacial weakness, the smile looks more like a snarl, and whistling or drinking through a straw becomes difficult or impossible. When facial muscle weakness is owing to LMN dysfunction, as in amyotrophic lateral sclerosis (ALS), fasciculations may be seen.
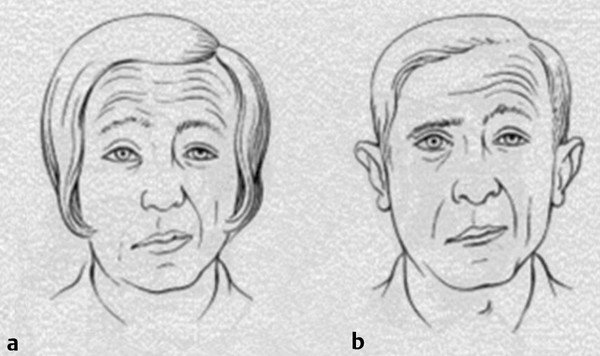
A key clinical point with respect to facial weakness is the distribution of the deficit. In a typical UMN lesion, such as a stroke, the muscles below the eyes are generally much weaker than those of the forehead and of eye closure ( ▶ Fig. 17.4a). In contrast, LMN weakness, as in Bell’s palsy, generally involves all ipsilateral muscles ( ▶ Fig. 17.4b).
Fig. 17.4 Facial nerve weakness. (a) Central or upper motor neuron-type facial palsy demonstrating sparing of the upper facial muscles. (b) Peripheral or lower motor neuron-type facial palsy showing involvement of muscles of the upper and lower face.
Weakness of the lower facial muscles result in dysarthria. Notably, the cheeks flutter when talking, there is drooling with speaking or eating, and the person has difficulty with uttering “puh.” Excessive drooling during meals or sleep predisposes the individual to aspiration.
Acoustic Nerve (CN VIII)
Cranial nerve VIII provides innervation for the cochlea and the end organs of the vestibular apparatus. Thus, this nerve provides the ability to hear and sense vertical, horizontal, and rotatory accelerations. Hearing may be tested at the bedside by having a patient listen for the ticking of a watch or recognize whispered words. A tuning fork is used to test whether air conduction is greater than bone conduction, as is normal. Vestibular function may be glimpsed by observing the eyes for movements in primary gaze or during pursuit movements. Detailed vestibular function testing is beyond the scope of this chapter.
Glossopharyngeal Nerve (CN IX)
The ninth cranial nerve supplies somatic sensation to the middle ear and the sense of taste to the posterior third of the tongue. The stylopharyngeus is the sole muscle innervated by this nerve. This muscle raises and dilates the pharynx. Testing of CN IX is accomplished by testing the gag reflex. A cotton-tipped applicator or tongue blade that gently touches the posterior wall of the pharynx should elicit elevation and constriction of the pharyngeal musculature, as well as retraction of the tongue. Although the afferent arc of this reflex (input) is carried through CN IX, the efferent function (output) is conducted through CN X. Some people, however, do not gag, so this should be considered unequivocally abnormal only if the gag is lost unilaterally.
Vagus Nerve (CN X)
The vagus nerve is a long and complex structure. It provides motor and sensory innervation to the palate, pharynx, and larynx. Additionally, it contains visceral sensory information from, and parasympathetic innervation to, thoracic and abdominal viscera. Also, CN X innervates some of the taste receptors on the posterior tongue and pharynx.
The thoracic and abdominal aspects of vagus function are not particularly relevant to speech and are not covered further. Functionally, the vagus nerve is extremely important for both swallowing and phonation, innervating most of the muscles involved with speech and swallowing except for the stylopharyngeus (IX) and tensor veli palatini (V). For the SLP, the three relevant branches of the vagus are the pharyngeal, the superior laryngeal, and the recurrent laryngeal nerves. After these segments have branched, the vagus nerve is concerned primarily with the viscera of the thorax and the abdomen.
Shortly after exiting the skull, CN X divides into branches. The pharyngeal branch descends to the inferior pharyngeal constrictor where it mingles with the glossopharyngeal nerve and external branch of the superior laryngeal nerve as the pharyngeal plexus. From this plexus, efferent fibers innervate all of the muscles of the palate and the pharynx, except those noted above.
The superior laryngeal nerve, the second important branch of CN X, divides into internal and external branches. The external laryngeal nerve supplies the inferior pharyngeal constrictor and the cricothyroid muscle. The cricothyroid muscle controls pitch by lengthening the vocal cords. The internal laryngeal nerve is a pure sensory nerve that receives mucosal sensory information from the pharynx down to the level of the epiglottis, the aryepiglottic folds, and the arytenoid cartilages. It also contains proprioceptive information from the muscle spindles of the vocal apparatus.
The third relevant branch of the vagus is the recurrent laryngeal nerve. Both the left and right recurrent branches are so named because after descending to the level of the branching great vessels in the mediastinum, they double back on themselves and ascend to the larynx. The right recurrent laryngeal loops under the right subclavian artery and the left loops beneath the arch of the aorta. Both recurrent laryngeal nerves innervate all intrinsic muscles of the larynx except for the cricothyroid.
Several functions of the vagus nerve are easily tested during the neurologic exam. Inspection of the palate reveals it to look lower and less archiform on the unilaterally weak side. When the patient says “ah,” there will be deviation to the normal side. The gag reflex will be reduced or abolished on the affected side. Unless palatal weakness is bilateral, hypernasality or nasal regurgitation of liquids during swallowing may not be noticeable. In bilateral weakness of the palate, speech becomes hypernasal and there is nasal regurgitation of liquids during swallowing. This form of dysphagia can lead to aspiration and aspiration pneumonia.
Normally, the vocal folds are abducted during inspiration and adducted during phonation and coughing. With unilateral abductor weakness, the voice may be hoarse but there will not usually be dyspnea. With bilateral abductor weakness, there is often severe dyspnea and inspiratory stridor. The voice may be hoarse but because both vocal folds can still be adducted, speech will not be severely affected. In a complete unilateral LMN lesion, the vocal fold lies motionless in midabduction. The voice is low-pitched and hoarse, but phonation may not be affected as much, as the normal cord may be able to cross the midline. With bilateral LMN weakness, there is inspiratory stridor, dyspnea, and loss of phonation.
Accessory Nerve (CN XI)
The eleventh cranial nerve is derived from anterior horn cells from the upper four or five cervical segments. Its fibers enter the skull through the foramen magnum, travel briefly with the vagus nerve, and ultimately innervate the sternocleidomastoid muscle (SCM) and the upper part of the trapezius (TPZ) muscle (the lower part of the TPZ is innervated by the third and fourth cervical roots through the cervical plexus).
Testing the SCM is achieved by asking the patient to turn the head away from the muscle as it is being palpated. The shoulder will appear depressed in repose and will wing somewhat with TPZ weakness. This will be accentuated with the arm abducted. Also, the TPZ is needed to elevate the arm above horizontal because the supraspinatus and deltoid muscles can only abduct the arm to approximately horizontal. To test muscle strength of the upper part of the TPZ, patients should be asked to shrug their shoulders.
Hypoglossal Nerve (CN XII)
The twelfth cranial nerve is a mixed nerve that innervates the tongue. The nucleus lies in the medulla beneath the floor of the fourth ventricle. It innervates all muscles of the tongue except the palatoglossus, which is innervated by the vagus. The sensory portion of the hypoglossal nerve is concerned chiefly with tactile information and is therefore important for chewing, swallowing, and articulation.
The motor function of CN XII is tested by asking the patient to protrude the tongue. Unilateral lesions of CN XII cause ipsilateral wasting (atrophy or loss of muscle bulk), deviation of the tongue toward the weak side, and fasciculation. To test the patient’s tongue strength, he or she is asked to push the tongue against the inside of the cheek while the examiner resists the pressure. With practice, the examiner should develop an appreciation of what is within the normal range. With UMN lesions, tongue movements are slower and weaker, particularly with lateral extension. In severe bilateral UMN lesions, as in ALS, the tongue may have relatively good bulk but is essentially immobile with attempted volitional movement. However, greater degrees of tongue movement may become apparent when the patient gags or yawns. This dissociation between volitional movement and reflex movement indicates that although substantial loss of the corticobulbar tracts may have occurred, the intrinsic bulbar reflex pathways are still relatively intact.
Patients with CN XII dysfunction have difficulty with consonant pronunciation, with utterance of “tuh” and “kuh,” as opposed to “puh,” and can have varying degrees of dysphagia because of poor coordination of swallowing.
17.3.3 Motor Examination
The goal of the motor examination is to assess the function of the motor unit and the various direct and indirect motor pathways. For the ambulatory patient, this begins with inspection of limb posture and a visual assessment of muscle bulk. For example, fixed postures across joints (contractures) may be indicative of long-standing lesions of the UMN. The presence of hammer toes generally indicates long-standing LMN loss of toe extensors leading to unopposed toe flexion. In essence, one’s resting posture is an unfailing indicator of the combined descending forces acting through the motor unit.
After inspection and palpation of limbs and muscles at rest, muscle tone is tested by passively stretching them. Increased muscle tone or hypertonicity can manifest as spasticity, the characteristic “clasp-knife” tone seen in corticospinal tract lesions, or “rigidity” as seen in Parkinson’s disease. Reduced muscle tone, or hypotonicity, is observed with acute cerebellar lesions. It is useful to observe the outstretched arms with the hands supinated (rotated outwardly) while the patient closes the eyes. A “pronator drift” (the drift of the hand to pronation) indicates a subtle corticospinal tract lesion. An upward or outward drift of the arm may be an indication of a sensory system lesion, demonstrating that with the eyes closed, the brain no longer “knows” where the limb is in space.
After the bulk and tone are assessed, strength and speed of individual muscles are tested. Numerous grading scales are used, but one of the most common ones is the Medical Research Council’s muscle strength scale, in which muscle strength is graded on a 0 to 5 scale (0 = no muscle contraction; 1 = flicker or trace of muscle contraction; 2 = active muscle movement, with gravity eliminated; 3 = active muscle movement against gravity; 4 = active muscle movement against gravity and resistance; 5 = normal strength). 1
17.3.4 Sensory Examination
Examination of the patient who has no major sensory complaint often is limited to gross testing of the primary sensory modalities. These are (1) detection of light touch with a wisp of cotton; (2) pain perception using a pinprick on the face and the distal aspects of the hands and feet; (3) evaluation of vibration sense over the fingers and toes using a 128- or 256-Hz tuning folk; and (4) test of proprioception or position sense in the fingers and toes by asking the patient to guess if the joint being moved is directed upward or downward.
On the other hand, examination of patients with sensory complaints is more detailed and is aimed at determining distribution, nature, and degree of sensory dysfunction, and whether the sensory symptoms are related to disturbances in the primary or secondary sensory modalities (i.e., when the sensory cortex or the thalamocortical radiations are affected.) In such instances, “primary” sensations of light touch, pinprick, and vibration may not be impaired while integrative sensations are (e.g., two-point discrimination, graphesthesia). Two-point discrimination is tested with the two points of a compass, for example. The patient is asked if the examiner has touched the skin with one or two points. Normally, we can detect two distinct points separated by several millimeters on the fingertips. For a shorter distance, the two points are experienced as one. On the back or trunk, however, a several-centimeter distance or longer is needed to make this distinction. Another test of integrative sensation is the ability to detect letters or numbers written on the palm or fingertips; this is graphesthesia. Finally, the ability to recognize objects placed in the hands by virtue of their shape and texture is stereognosis. These abilities are also impaired with sensory lesions at, or rostral to, the thalamus.
17.3.5 Reflexes
The examination of reflexes provides important objective information about the integrity of the nervous system in two ways. First, most reflexes are mediated by monosynaptic or oligosynaptic (a few synapses) pathways that have afferent arcs outside the CNS, that enter the CNS, and that have efferent arcs that mediate a motor function in the periphery. Thus, the integrity of the nervous system in a “horizontal” sense can be assessed with the tap of a tendon.
For example, tapping on the triceps tendon at the elbow produces an afferent volley in the radial nerve that enters the spinal cord through the C7 dorsal root. The large sensory nerve fibers mediating the reflex synapse directly on lower motor neurons also in the C7 spinal cord segment. These are induced to fire and an efferent volley goes down some of the LMN destined to innervate the triceps, producing the triceps contraction. If the peripheral nerve, the dorsal or ventral root, or the spinal cord segment itself is damaged, the reflex may be abolished or reduced.
Another type of information reflexes provide is in a vertical sense. The briskness of a reflex is determined by the descending corticospinal tract tone. A UMN lesion produces hyperreflexia (increased reflex) below the level of the lesion. In other words, a midthoracic spinal cord lesion would produce hyperreflexia, or exaggerated reflexes, in the legs, but the reflexes in the arms and cranial innervated structures would remain normal. Similarly, a unilateral corticospinal tract lesion at the level of the frontal lobe would be expected to produce hyperreflexia in all contralateral muscles that receive its projections. This is the most common scenario following a cerebral infarct producing contralateral hemiparesis.
17.3.6 Coordination
Coordination primarily is a function of the cerebellum. Acute cerebellar hemisphere lesions produce a constellation of signs ipsilateral to the lesion: hypotonia, ataxia, tremor, and a slight amount of weakness. These signs often improve over time. Two common tests of cerebellar function are the “finger–nose–finger” test (in which patients alternately touch the examiner’s finger, their own nose, the examiner’s finger, etc.), and the “heel–shin” test (in which patients carefully slide their heel along the contralateral tibia from knee to ankle). The cerebellum is vital for the motor control of coordinated movement. With cerebellar lesions, there is decomposition of the planned movement. In the heel–shin test, for example, one might see jerky movements that represent abnormalities in the degree, duration, and timing of the contraction of the quadriceps (as the heel slides distally), and the relaxation of the hamstrings and iliopsoas muscles.
17.3.7 Gait and Stance
The assessment of gait and station is an essential part of the neurologic examination, which may provide significant information about many different areas of the nervous system. Standing upright is possible only with adequate proprioceptive information concerning the location of the body and limbs in space, muscular power to maintain the erect posture, and vestibular and visual input to centers involved in “righting reflexes.” Locomotion involves voluntary sequential firing of leg flexors and extensors as well as involuntary or “unconscious” input to the arms, legs, and trunk muscles from cortical structures like the frontal lobe.
A detailed discussion of gait physiology and the full spectrum of abnormal gaits can be found elsewhere, 2 but some illustrative examples are worth highlighting. In a hemiparetic gait, the involved leg is slow and weaker, particularly in movements involving flexion of the hip and dorsiflexion of the foot. This results in patients’ circumducting the leg in an attempt to avoid a premature strike of the toe. There is decreased arm swing on the involved side, and the arm may be held flexed and abducted.
Sensory ataxic gait is caused by polyneuropathy—damage to multiple peripheral nerves—or spinal cord disease. Without having a precise knowledge of the position of the feet and limbs, the patient needs a widened stance. The individual movements of the legs are exaggerated in force and degree. The visual system may provide a partial compensation for these deficits; that is, watching the ground may help compensate for the loss of proprioceptive information. Consequently, under these circumstances, the gait worsens in low light situations or when the eyes are closed. A similar gait is seen in disease affecting portions of the cerebellum that normally coordinate gait.
Parkinsonian gait is fairly stereotypic. The patient stands somewhat stooped with the head forward and the arms slightly flexed. The steps are shortened and often shuffling. There is decreased arm swing and the patient may “festinate,” that is, involuntarily speed up and begin to shuffle forward (propulsion) or backward (retropulsion). In severe parkinsonism, these abnormalities, along with loss of righting reflexes, significantly impair independent ambulation.
Myopathic gait occurs when there is sufficient weakness of the hip flexors and other muscles of the pelvic girdle. This type of gait is typically waddling. Normally, when one is walking, the gluteal muscles on the weight-bearing leg elevate the contralateral hemipelvis slightly to allow the free forward swing of the nonweight-bearing leg. If these muscles are weak, when the patient walks, the pelvis tilts downward on the side of the free-swinging leg, producing a waddling from side to side. This same abnormality occurs in neurogenic weakness if it prominently affects the hip girdle muscles, particularly the gluteus medius.
17.4 Common Neurologic Testing
A detailed neurologic history and systematic neurologic examination provide the majority of information needed to formulate a differential diagnosis and establish a working diagnosis of the patient’s presenting complaints. Neurologic testing refines our diagnostic accuracy. Imaging studies, such as computed tomography (CT) and magnetic resonance imaging (MRI), are cornerstone ancillary neurodiagnostic tests. A detailed description of these tests and their indications is found elsewhere in the book.
17.4.1 Electrophysiologic Tests
Electroencephalography (EEG), magnetoencephalography (MEG), evoked responses, and electromyography (EMG) are testing modalities based on the fact that nerve cells and muscle are electrically—electromagnetically—active tissues. They maintain measurable voltages (potentials) across their membranes at rest and generate changes in these potentials as a means of integrating and communicating information to and from other cells. To date, MEG largely remains a tool used for basic research, particularly in cognitive research, epilepsy, and migraine. The utility of MEG as a diagnostic test is not fully explored, except in surgical localization, and therefore is not covered here. Conversely, EEG and EMG often are used for neurodiagnostic purposes. Lastly, evoked responses (visual, auditory, and somatosensory), once commonly used in neurology, have been largely abandoned in favor of more accurate and precise neurodiagnostic techniques. Evoked response testing is not covered in this chapter.
Electroencephalography
An EEG is a record of brain electrical activity generated by electrodes positioned on the surface of the head or scalp. The electrodes are placed in a standardized format that uses common anatomical landmarks as points of reference ( ▶ Fig. 17.5). The recorded activity directly reflects the activity of the cortex below, specifically the postsynaptic potentials of the large vertically oriented pyramidal cells. Indirectly, though, structural or functional abnormalities of the underlying white matter or of the thalamocortical projections may produce characteristic changes as well.
Fig. 17.5 Outline of the head, seen from above, shows the standardized placement of electrodes according to the International 10–20 system. The EEG record indicates a normal 16-channel EEG recorded in an anterior to posterior bipolar montage.
In general, a localized “spike and wave,” “spikes,” or other paroxysmal activities can be suggestive of a seizure focus. The electroencephalographer looks for either the maximal amplitude of the spike discharge or phase reversal of the spike between adjacent electrodes to localize the focus on the underlying brain. Some EEG patterns are highly reliable signatures of a given epileptic syndrome, and therefore highly predictive of effective treatment with one or another class of antiepileptic drugs (AED). Other EEG patterns are suggestive of heritable, though benign and self-limited, seizure disorders. In others, the pattern suggests a severe disorder of brain function and portends a dismal future for the patient. In epileptic patients studied repeatedly over time, at least 90% ultimately demonstrate epileptiform EEG activity 3 ( ▶ Fig. 17.6a). Other factors that improve the diagnostic yield of an EEG in the evaluation of spells of altered consciousness include 24-hour sleep deprivation, sleep, hyperventilation, and photic stimulation (strobing lights).
Fig. 17.6 Abnormal electroencephalographic (EEG) recordings. (a) A focal-onset secondary generalized seizure. Note that rhythmic activity begins in the right temporal area and subsequently spreads to involve the whole brain. The sharp, dark lines on the right side of the record are motor/muscle artifact caused by the onset of tonic-clonic activity in the muscles of the head and face. (b) EEG in severe diffuse encephalopathy. The bottom line is the electrocardiogram (ECG). Note that the record is characterized by slow irregular activity that is unresponsive to loud clapping and calling the patient’s voice. Note also that some of the sharper waveforms are actually ECG artifact.
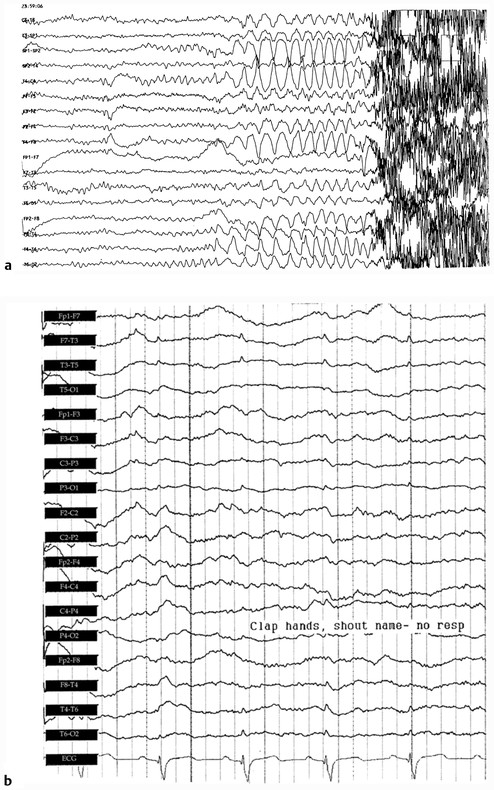
EEG is principally used to classify and to prognosticate seizure disorders. Also, an EEG is used in the diagnostic evaluation of spells of altered consciousness for which the cause may not be obvious on routine history and physical examination ( ▶ Fig. 17.6b). Furthermore, EEG may differentiate an epileptic from a nonepileptic spell. EEG sometimes is used in confirmation of brain death (i.e., the irreversible cessation of brain function). In this circumstance, a “flat” or isoelectric record is obtained. 4, 5 Finally, EEG is used in surgical monitoring of cerebral function, for example, during carotid endarterectomy.
Nerve Conduction Studies and Electromyography
Conventionally, EMG is a test constructed of two parts: the nerve conduction study and the needle exam (NE) or the electromyogram. Nerve conduction studies assess peripheral motor and sensory nerve and neuromuscular junction (NMJ) functions.
Nerve Conduction Studies (NCS)
Peripheral nerves are found in a wide variety of diameters but only the large myelinated—hence rapidly conducting—fibers are measured using most conventional EMG techniques. Small myelinated and unmyelinated fibers generate such small signals that, without special techniques, they are lost as “noise.”
To understand NCS changes seen in pathological conditions, it is useful to imagine a plastic-covered telephone wire as a model for a myelinated nerve fiber. The central copper wire is analogous to the axon. It carries the information encoded in the electrical current; if it is severed, the call is lost. The plastic coating, analogous to the myelin sheath synthesized peripherally by the Schwann cell, provides for isolation and insulation of the line. If the plastic sheath is damaged, there can be cross talk or “shorts” between wires. Additionally, in myelinated nerve fibers (but not phone lines), the relationship between the normal sheath and the axon it envelops is vital for normal conduction. If the myelin sheath is damaged, conduction in the axon may slow or cease altogether; this is known as conduction block.
NCS are generally performed by percutaneous (over the skin surface) stimulation of an accessible portion of a peripheral nerve and simultaneous recording from a proximal or distal portion of the nerve or the muscle it innervates. In the case of sensory nerves, the recording is made with applied disk or ring electrodes directly above the nerve. When recording from motor nerves, disk electrodes are placed directly over the muscle innervated by the nerve. The resulting evoked response is actually generated by the muscle itself. Because of these differences, although sensory nerve action potentials are in the microvolt range, motor-evoked responses, representing the depolarization of many grams of electrically excitable muscle tissue, are in the millivolt range. The data obtained using these techniques include conduction velocity, terminal or distal latencies, and measurements of the sensory and motor-evoked response amplitudes.
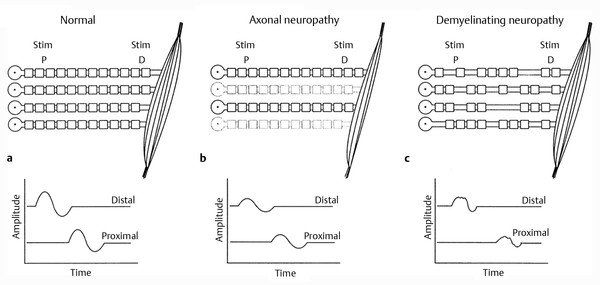
Disorders of peripheral nerves generate a fairly limited number of abnormalities on NCS despite the limitless number of insults or diseases that can affect them. ▶ Fig. 17.7a depicts characteristic abnormalities seen in common disease states. Two contrasting neuropathic (i.e. pathological states affecting the nerve) patterns are illustrated as examples. A summary of the NCS findings is shown in ▶ Table 17.2.
Fig. 17.7 (a) Stylized motor nerve conduction study. The four myelinated nerves are stimulated distally and proximally to yield the two traces shown below. The compound motor unit action potential (CMAP) is the summed electrical potential of the four individual motor unit action potentials; amplitude and latencies of responses are usually recorded. (b) Axonal neuropathy. With any lesion with axon loss, there is loss of muscle bulk and hence a loss of the summed individual action potentials. Note that the latencies and therefore the calculated conduction velocity are relatively spared. (c) Demyelinating neuropathy. Note that random multifocal loss of internodes of myelin leads to the unpredictable slowing in individual nerve fibers. The third fiber from the top has lost too many internodes to conduct and fails to activate its muscle fibers. The resulting waveforms are therefore delayed, “dispersed,” or notched because of the temporal dyssynchronization of the individual motor unit action potentials, and of lower amplitude because of the complete loss of conduction of one of the nerve fibers.
Type of Neuropathy | Terminal latency | Conduction Velocity | Amplitude |
Axonal | Normal to slightly shorter | Normal to slightly slower | Lower |
Demyelinating | Prolonged | Slower | Normal to slightly lower |
In an axonal neuropathy, as individual axons are lost, the number of individual elements that participate in the conduction is decreased and the resulting amplitude is diminished. Although ▶ Fig. 17.7b illustrates a motor fiber with its connection to the muscle, the situation is exactly analogous to a sensory conduction. Because axonal disease does not involve the Schwann cell (i.e., the myelin sheath), the distal latency and conduction velocity are relatively spared.
NCS findings ( ▶ Fig. 17.7c) in a demyelinating neuropathy, such as Guillain-Barré syndrome, show delay of individual motor unit action potentials with proximal stimulation. This has several consequences. First, the terminal latency can be delayed, and the resulting conduction velocity will be slowed. Second, as the individual motor unit action potentials are delayed, the resulting compound motor unit action potential (CMAP) is less synchronous with respect to time. As the individual motor unit action potentials are temporally dispersed, their positive and negative phases begin to cancel electrically. This results in a CMAP that is of lower amplitude and potentially spread out with respect to time, or “temporally dispersed.” A conduction block of that axon may take place if the process causing the demyelinating neuropathy damages a sufficient portion of the myelin sheath. In essence, while still alive and functioning in many ways, the individual motor fiber may cease to participate in voluntary contraction of the muscle. This manifests clinically as weakness.
Tests of Neuromuscular Transmission
Disorders of neuromuscular transmission are of great clinical relevance to the neurologist and SLP. The most common and prototypical disorder of neuromuscular transmission is myasthenia gravis (MG).
Several tests of neuromuscular transmission are used to diagnose MG or to measure the effect of therapy; repetitive nerve stimulation (RNS), known as the Jolly test, and single-fiber EMG (SFEMG) are the most common. 6 Before describing these tests, a brief overview of the physiology of neuromuscular transmission will be helpful. When a motor nerve is stimulated, a wave of depolarization proceeds toward the nerve terminal. Close to the terminal, the motor axon branches into many small nerve endings, each of which ends in close apposition to a single muscle fiber. When the wave of depolarization proceeds to the end of each nerve terminal, the resulting voltage change induces an influx of calcium from the extracellular space. The rise in calcium concentration at the nerve terminal causes the release of many membrane-bound packets of the neurotransmitter acetylcholine (ACh) into the synaptic cleft. The ACh quickly diffuses across the cleft and binds to postsynaptic ACh receptors (AChRs) on the muscle fiber. When a molecule of ACh binds to the AChR, a conformational change occurs in the receptor that causes a small depolarization of the muscle membrane to take place.
There are many AChRs on the muscle membrane, and if enough are stimulated, the small depolarizations of the muscle membrane summate and cause the muscle fiber to reach threshold. At threshold, a muscle fiber action potential takes place. As the muscle fiber depolarizes along its whole length, it begins to contract and generate force, a process known as excitation-contraction coupling.
One other fact must be appreciated to understand the physiology of fatigable weakness as observed in MG. When the nerve terminal depolarizes and the waiting packets of ACh are released into the synaptic cleft, there is a normal diminution of the number of packets released with each successive stimulation. If the first stimulation induced the release of, say, 100 packets, by the fourth stimulation perhaps 50 would be released. Under normal circumstances 50 would be more than enough to produce a muscle action potential, but in MG, if a large percentage of AChRs have been destroyed or are nonfunctional because of antibody attack, the 50 packets of ACh may be inadequate to cause the depolarization of the muscle fiber. The failure to depolarize is manifested clinically as weakness.
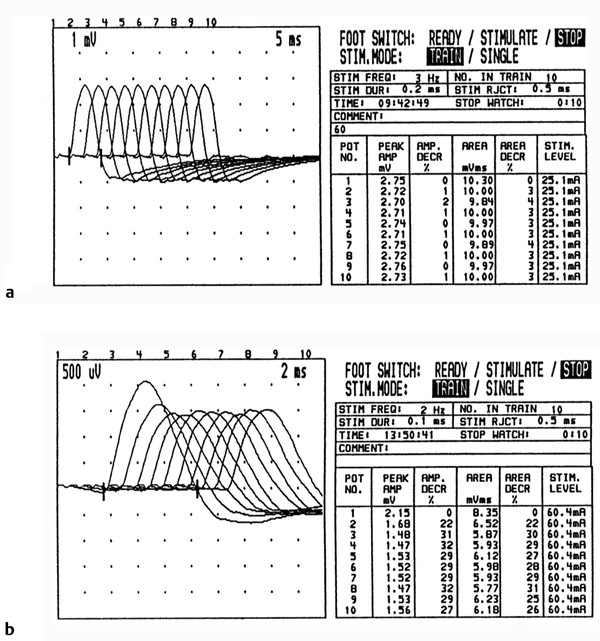
In the RNS technique, stimulating electrodes are placed on a motor nerve and recording electrodes on a muscle supplied by the motor nerve. Four to 10 shocks at 2 to 3 Hz (aptly named repetitive stimulation) are given, and the amplitude of the evoked motor responses is measured at the first and fourth stimulation. Amplitude decrements of greater than 10% are considered pathological and suggestive of a defect in neuromuscular transmission ( ▶ Fig. 17.8).
Fig. 17.8 Repetitive nerve conduction study (Jolly test). (a) Normal test. The CMAPs were recorded from the hypothenar muscle while the ulnar nerve was stimulated at 3 Hz. Note that each waveform differed in amplitude or area by less than 4%. (b) Myasthenia gravis. This Jolly test was performed on a patient with myasthenia gravis (at 2 Hz in this case). Note that with repetitive stimulation there is a marked reduction of amplitude, most prominent from the first to the second potential and reaching a nadir with the fourth stimulation. See the text for an explanation of this phenomenon.
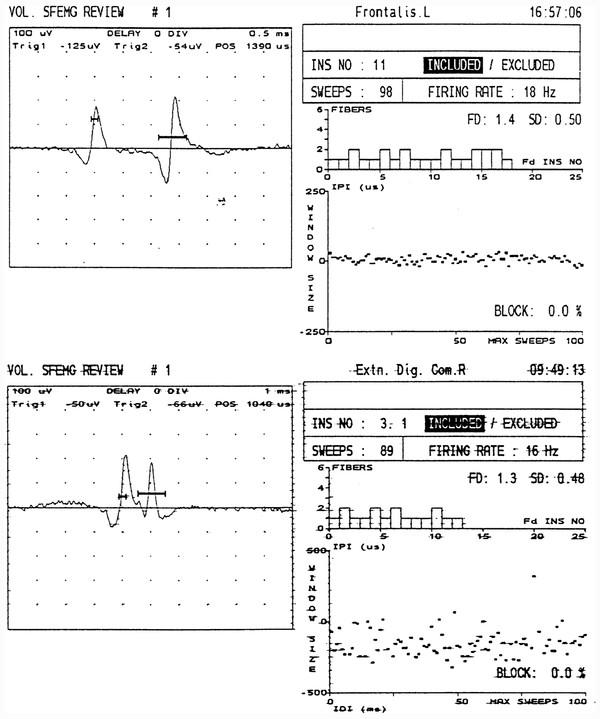
Single-fiber EMG is a more sensitive and more complex test used to detect defects of neuromuscular transmission. The patient is asked to barely activate a muscle while a special needle electrode is maneuvered until two spike potentials firing “simultaneously” are identified. With normal neuromuscular transmission, the variation in time with consecutive discharges between the firing of the first potential and the second is extremely small, on the order of 20 to 45 microseconds ( ▶ Fig. 17.9). The measurement of this variability is called “jitter.” The reduced reliability of neuromuscular transmission in MG leads to an increased variability or jitter in the firing of a given muscle fiber action potential with respect to a second fiber from the same motor unit. Sometimes the firing of the second fiber fails altogether, an event termed “blocking.” SFEMG is abnormal in greater than 95% of patients with generalized MG.
Fig. 17.9 Single-fiber EMG (SFEMG). (a) Normal recording from the frontalis muscle. The two spikes represent the potentials from two individual muscle fibers of the same motor unit. The bold horizontal lines crossing the potentials above the baseline represent “triggers” (voltage thresholds for the computer program to recognize) for the display of the potential and the calculation of when the second spike fires relative to the first. From the interpotential interval (IPI) seen in the graph in the lower right, a calculation is made of the “jitter,” or variability between the firing of the first and second muscle fibers. Most of this variability results from neuromuscular transmission. In this case, the jitter was 11 sec (normal is 45 sec). (b) Myasthenia gravis (MG). Abnormal SFEMG from a patient with MG demonstrating jitter of 99 sec.
Electromyography
The needle exam (NE) is an essential part of EMG, providing vital information on the function of the motor unit. If there is weakness from a disorder of the motor unit, the NE should be able to detect it. Even in weakness owing to disorders of the UMN, abnormalities of diagnostic significance are often apparent (e.g., changes in motor unit firing patterns or frequencies).
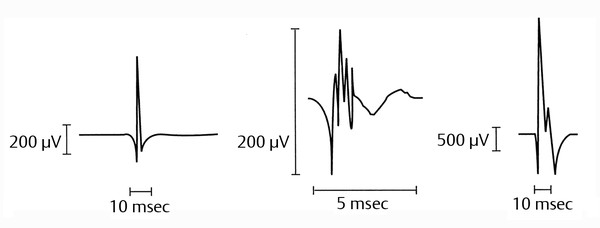
The NE involves the insertion of a small needle electrode into the muscle of interest. The tip of the needle acts as the recording electrode from within the muscle, and the activity is displayed on a video monitor. EMG machines assign sounds to the waveforms based on their morphologies. The question asked of the electromyographer determines which muscles will be sampled. Spinal and extremity muscles commonly are tested. Also, facial and sometimes laryngeal muscles (percutaneous and endoscopic testing) can be sampled. ▶ Fig. 17.10 illustrates characteristic NE findings.
Fig. 17.10 Electromyograms in health and disease states. (a) Normal motor unit. (b) Myopathic motor unit: Note the low amplitude, short duration, and polyphasic characteristics. (c) Neurogenic motor unit: Note the high amplitude, long duration, and polyphasic characteristics.
Another method of directly obtaining information regarding the electrical activity of muscle during the EMG is by recording muscle surface activity (surface EMG, SEMG). In SEMG, the active recording electrode is placed over the belly of the muscle and the reference electrode is placed along a tendon (tendinous insertion). SEMG is insensitive to whether a muscle is denervated or whether the muscle has myopathic (pathology of the muscle itself) or neuropathic features, but it is able to provide other kinds of useful information. SEMG is used in several clinical and research situations. It is particularly useful in “central EMG,” studying the patterns and timing of muscle activation in movement disorders or other disorders of central motor control. SEMG can also be used to provide auditory or visual signals for biofeedback.
17.4.2 Ultrasonography
Vascular ultrasonography is a useful technique in neurodiagnostics as it allows visualizing and measuring blood flow in normal and diseased cerebral vessels. During a vascular ultrasound, the ultrasound machine transmits sound waves through tissues (e.g., skin, soft tissue, and carotid artery wall and lumen in case of carotid Doppler study). Blood cells that are moving within the vessels transmit sound waves back to the measuring probe (transducer). The strength of the reflecting signal depends on the velocity of the blood column flowing through the vessel being examined. Reflected sound waves can be transformed into images (B-mode) or the velocity of the blood flowing through the vessel can be measured (M-mode).
Cerebral vascular ultrasonography once was a cornerstone neurodiagnostic test in cerebrovascular diseases. Advances in CT (e.g., CT angiography) and MRI (e.g., MR angiography) led to a significant decline in the use of cerebrovascular ultrasonography. Vascular imaging techniques are discussed elsewhere in the book.
Carotid Doppler
Carotid Doppler is a noninvasive, painless, and harmless neurovascular diagnostic procedure that is completed at bedside or in a vascular diagnostic laboratory. Carotid Doppler studies visualize the carotid arteries (external, internal, common) and determine the degree of narrowing or stenosis in those arteries, if present. Visualization allows the examiner to identify luminal narrowing (reduced diameter of the artery) and vascular wall abnormalities (e.g., plaque). Measures of blood flow velocities inside the internal, external, and common carotid arteries help in determining normalcy, narrowing, or occlusion of the insonated artery. Finally, carotid Doppler studies identify flow direction in the vertebral arteries, which normally is anterograde (i.e., blood is flowing toward the brain and away from the recording probe.) Patients with the condition known as subclavian steal syndrome may have reversal of flow (retrograde flow) in the vertebral artery ipsilateral to the narrowed subclavian artery.
Transcranial Doppler
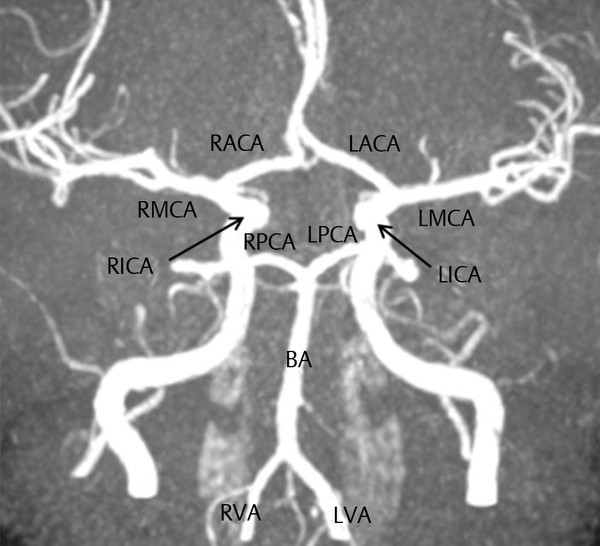
Transcranial Doppler (TCD) ultrasonography measures cerebral blood flow indirectly by assessing local cerebral blood flow velocity (CBF-V) in the major cerebral arteries ( ▶ Fig. 17.11). TCD has the advantages of being noninvasive, portable, repeatable (providing continuous measurements), and inexpensive. Disadvantages of TCD are (1) it can measure CBF-V only in major cerebral arteries; (2) it has high operator-dependence; and (3) it depends on easily insonated bone windows. 7
Fig. 17.11 Magnetic resonance angiogram of the circle of Willis showing arteries that typically are insonated by transcranial Doppler (TCD). R, right; L, left; ACA, anterior cerebral artery; BA, basilar artery; MCA, middle cerebral artery; PCA, posterior cerebral artery; VA, vertebral artery. Adapted from http://commons.wikimedia.org/wiki/File:Circle_of_Willis_-_MRI,_MIP_-_Anterior_projection.png
TCD is used primarily in the evaluation of ischemic and hemorrhagic cerebrovascular disease. Furthermore, TCD can assist in the evaluation of patients with suspected malformations, such as cerebral aneurysm or arteriovenous malformations (AVM). Also, TCD can assist in the evaluation of cerebral vasospasm, which may be posttraumatic or may follow aneurysmal subarachnoid hemorrhage (SAH). Lastly, TCD is complementary to the neurologic examination and EEG in assessing brain death. 8 The most acceptable indications for TCD are screening of children and adolescents with sickle cell disease to assess for risk of ischemic stroke, and detection and monitoring of vasospasm after SAH. 9
17.4.3 Lumbar Puncture
Lumbar puncture (LP), which is also known as spinal tap, involves the insertion of a spinal needle in the lower lumbar region (back) in order to draw cerebrospinal fluid (CSF) for diagnostic—and sometimes therapeutic—purposes. An LP is performed under local anesthesia at bedside or under X-ray guidance (fluoroscopy). First, the patient or legal proxy is asked to consent to the LP after full description of the procedure, its indication(s), risks, and benefits. Then, the patient is positioned in the desired position (preferably the lateral recumbent or fetal position in order to accurately measure CSF pressure). The skin where the needle insertion is planned subsequently is cleaned and anesthetized using local anesthetic, such as lidocaine. Then, the examiner advances the spinal needle carefully into the back (typically the L4–L5 disk space and sometimes the L3–L4) until a “pop” is felt after encountering resistance. This indicates that the needle has penetrated the dura and it is in the subarachnoid space where CSF flows. Once CSF is dripping from the spinal needle (normally CSF is clear and colorless), the examiner measures CSF pressure and obtains several tube samples for diagnostic purposes. Finally, the needle is carefully withdrawn and the patient is allowed to rest flat for 2 or more hours. Prolonged bed rest (i.e., beyond 2 hours) is not associated with added benefit, such as reducing the risk of post-spinal headache.
A diagnostic LP is obtained when meningitis (e.g., bacterial, viral, tuberculous, fungal, or syphilitic) or encephalitis is suspected and as a complement to early CT scanning in suspected subarachnoid hemorrhage. A diagnostic LP also is obtained in suspected meningeal carcinomatosis, multiple sclerosis, and Guillain-Barré syndrome. A diagnostic and therapeutic LP is used in cases of benign or idiopathic intracranial hypertension (also called pseudotumor cerebri), where the patient presents typically with headache and papilledema (swelling of the optic disc on funduscopy), and imaging studies do not show any evidence of space-occupying intracranial lesion. Lastly, a therapeutic LP is obtained in suspected cases of normal pressure hydrocephalus (NPH), where patients typically present with the triad of cognitive decline, gait abnormalities, and urinary incontinence. In early cases of NPH, drainage of sufficient CSF fluid can improve patients’ symptoms, particularly cognitive deficits.
Contraindications to an LP are suspected raised intracranial pressure, a severe bleeding diathesis (e.g., very low platelets or when a patient is receiving anticoagulant therapy), infection at the planned site of a spinal tap, tethered cord (spinal cord extends into lower lumbar and sacral regions), and suspected complete spinal block above the planned level of the tap.
An LP is safe when properly performed. Most common complications of a spinal tap include headache, backache, and nerve root or radicular pain. Infections, bleeding, and cerebral herniation are rare complications of an LP that can be avoided if proper techniques and indications are followed.
17.5 Ten Common Neurologic Presentations an SLP Should Know
A comprehensive review of neurologic diseases and their management is beyond the scope of this chapter. The following topics are meant to introduce SLPs to common neurologic manifestations or diseases they might encounter in their practice. The majority are seen in an inpatient setting.
17.5.1 Change in Mentation: Focus on Delirium and Dementia
Delirium
Delirium is a transient—usually reversible—condition that clinically manifests as a constellation of neurologic and psychiatric symptoms and signs. Delirium is a common presentation in the emergency department and on medical and surgical wards. 10 It is estimated that >30% of hospitalized patients over 70 years old and over 70% of elderly patients in intensive care units experience delirium. 11 SLPs often are consulted on delirious patients for speech and language derangements and for evaluation of dysphagia and risk of aspiration and aspiration pneumonia.
Causes and Risk Factors
Delirium can be caused by a variety of factors and often has multiple underlying causes 12 ( ▶ Table 17.3). Drugs 13 and infections are among the most common causes of delirium in hospitalized and long-term care patients. Generally, drugs with short half-lives are more commonly associated with delirium. Over-the-counter medications (e.g., diphenhydramine), prescription drugs, and illicit substances (e.g., cocaine) can cause delirium. Also, patients with renal insufficiency are particularly prone to delirium when they take medications that are cleared by the kidneys. Lastly, the elderly often use combinations of drugs, and a drug-drug interaction can be a factor in causing delirium.
Cause | Examples |
Drugs | Narcotics (e.g., meperidine), diphenhydramine, neuroleptics (e.g., haloperidol), sedative-hypnotics, antiepileptic drugs (e.g., phenobarbital), antihypertensive β–blockers (e.g., propranolol) |
Eyes and ears | Sensory deprivation from hearing loss or visual loss |
Low oxygen states | Congestive heart failure, respiratory insufficiency |
Infections | Urinary tract infection, pneumonia |
Retention | Severe constipation; urinary retention |
Intoxication | Alcohol excess, illicit drugs |
Uncontrolled pain | |
Metabolic derangement | Hypo- and hypernatremia, hypo- and hyperglycemia, hypo- and hypercalcemia, dehydration, fluid overload, thyroid derangements |
Patients with renal insufficiency, alcohol abuse, or bleeding tendencies can develop a spontaneous subdural hematoma without antecedent head injury and can present in a delirious state without other neurologic complaints, such as weakness or headache.
Advancing age, dementia, multiple comorbidities, drug-drug interactions, prolonged hospitalization, intensive care setting, and multiple medication use are among the major risk factors for delirium. In the elderly, it is common to implicate multiple risk factors in inducing a delirious state. Also, delirium is common in critically ill patients, in postoperative patients after major operations, such as coronary artery bypass surgery (CABG) or total hip replacement, and after prolonged anesthesia.
Clinical Manifestations
Hallmarks of delirium are (1) impaired level consciousness (e.g., reduced awareness, inattention); (2) a change in cognition (such as memory deficit, disorientation, language disturbance) or the development of a perceptual disturbance that is not better accounted for by a preexisting, established, or evolving dementia; (3) a disturbance developing over a short period of time (usually hours to days) and tending to fluctuate during the course of the day; (4) evidence from the history, physical examination, or laboratory findings that the disturbance is caused by the direct physiological consequences of a general medical condition. 14
Often, individuals with delirium manifest fluctuating behavioral and neuropsychiatric symptoms (e.g., agitation, confusion, hallucinations, inattention, disorganized thinking, incoherent conversation) that are more pronounced late in the day or evening.
Management
Delirium is an acute confusional state and a medical emergency. As with any other neurologic emergencies, accurate—and early—diagnosis and effective management of delirium reduce associated morbidity and mortality rates. Identifying and correcting reversible causes, such as medications, metabolic derangements, and sensory deprivation, are important management steps. Supportive care measures include proper nutrition and hydration; ensuring regular and adequate sleep; effective treatment for pain, if present; management of constipation, which is common in elderly, particularly with prolonged immobility or postsurgically; providing a comfortable environment with warm ambient temperature, sufficient lighting during the day, dim lights at night; and placement of a clock and calendar in the room to assist with orientation. Hospital room changes and frequent changes in staffing should be avoided.
Physical restraints are to be avoided at all cost since they are associated with the development of, or exacerbation of, existing delirium. Also, it is a misconception that restraints can mitigate falls and reduce fall-related injuries. In fact, well-conducted studies have shown that use of physical restraints either increases or has no effect on fall rates, and fall-related injuries are more common when patients with delirium are restrained. 15, 16, 17
Pharmacotherapy for delirium often is unnecessary when supportive care is optimized. To date, there is no FDA-approved pharmacologic treatment for delirium, but limited use of antipsychotic drugs may be warranted when the patient exhibits disturbing hallucinations. Aberrant behaviors and severe agitation that pose a significant risk of physical harm—personal harm and risk to others—are best managed with sedatives, such as lorazepam. The goal of such treatments is comfort and mitigation of harm, and not sedation.
Complications
Prolonged length of hospital stay, with its associated increased risk of nosocomial complications, and higher rates of discharge to long-term care facilities are well-known complications of delirium. Patients with delirium are at higher risk of major medical complications, such as pneumonia, respiratory insufficiency, pulmonary edema, and myocardial infarction. 18 Lastly, delirium after major surgery is linked to higher rates of cognitive dysfunction, weeks to months postoperatively. 19
Dementia: Focus on Alzheimer’s Disease
Dementia is a progressive decline in memory functions with impairment in at least one other domain of cognition (e.g., speech) leading to impaired functioning. Alzheimer’s disease and vascular dementia are among the most common causes of dementia. Alzheimer’s disease accounts for over 60% of all dementing illnesses. Its prevalence is approximately 1% in 65-year-old people, and doubles every 5 years thereafter. It is estimated that the cost (direct and indirect) of Alzheimer’s disease approaches $200 billion. 20
Vascular or multi-infarct dementia has its highest prevalence in people 60 to 75 years old, occurs in people with significant cardiovascular and cerebrovascular risks (e.g., hypertension, diabetes, myocardial infarction), and manifests as cognitive impairment with varying degrees of focal neurologic signs and symptoms that depend on infarct locations. Management and complications of vascular dementia are not covered in this chapter.
Causes and Risk Factors
As mentioned previously, Alzheimer’s disease and vascular dementia are the most common dementing illnesses. 20, 21 Less common causes of dementia are listed in Table ▶ Table 17.4.
Risk factors for Alzheimer’s disease include Down syndrome, hypertension, history of head trauma, mild cognitive impairment (MCI, see below), a family history of dementia, age, and female gender. 21, 22, 23 Possible social factors implicated in increased risk of Alzheimer’s disease are low physical, mental, and/or social activities. Lastly, there is some evidence that dietary factors (e.g., reduced intake of vitamin E or D) may be linked to an increased risk of Alzheimer’s disease. 24, 25
Clinical Manifestations
Dementia can affect multiple functions in the brain, leading to a variety of symptoms and signs. Manifestations of dementia include the following:
Difficulty with learning and retaining information, resulting in symptoms like repetitiveness, trouble remembering conversations, and frequently misplacing items;
Problems with handling complex tasks, such as meal preparation, medication administration, and finances;
Impaired reasoning, resulting in difficulties with planning, problem solving, or following rules of social conduct;
Spatial disorientation, with signs like household disorganization and losing one’s way around familiar settings;
Language problems, with difficulties in word finding or following a conversation; and
Behavioral symptoms, such as being passive or less responsive, irritable, suspicious, misinterpreting visual or auditory stimuli, and hallucinations.
Classically, symptoms and signs of dementia, particularly in the case of Alzheimer’s disease, appear and progress depending on the stage of the illness. Early stages of dementia manifest as memory loss for recent events, changes in behavior and personality, and impairment in work/skills performance. In middle-stage dementia, symptoms become more noticeable and can include one or more of the following: significant decline in skills, difficulty identifying objects and understanding directions, confusion and disorientation, and worsening personality changes. In late stages of dementia, the individual loses basic skills, such as eating and drinking, weight loss manifests, the person loses the ability to ambulate independently, and there is loss of recognition of familiar people and environments.
Mild cognitive impairment is a condition of memory loss that is beyond what is expected with normal aging. Individuals with MCI persistently forget meaningful information but have minimal or no loss in their daily work or routines. Complex tasks that require complex reasoning, decision making, and planning (e.g., paying bills) may require longer time to complete and some errors may be committed. A significant number of patients with MCI develop Alzheimer’s disease as they age.
Management
First and foremost, it is essential to establish an accurate diagnosis of dementia and to establish whether the cognitive decline is related to an irreversible illness, such as Alzheimer’s disease, or is related to reversible causes ( ▶ Table 17.4).
Category (CNNSHOTIME) | Examples |
Cerebrovascular | Vascular dementia, post-intracerebral or subarachnoid hemorrhage |
Neurological, Neuropsychiatric | Alzheimer’s disease, parkinsonism (e.g., progressive supranuclear palsy, corticobasal disease, Lewy body disease), depression, normal pressure hydrocephalus, frontotemporal dementia |
Neoplastic | Primary and metastatic CNS neoplasm, paraneoplastic syndromes |
Systemic | Systemic vasculitis, chronic anemia, congestive heart failure, chronic hypoxia (e.g., chronic obstructive pulmonary disease, sleep apnea), chronic renal insufficiency, hepatic failure |
Hereditary | Familial dementias, hyperhomocystenemia |
Others | Deficiency syndromes (e.g., vitamin B12, thiamine) |
Traumatic | Traumatic brain injury, subdural hematoma |
Infectious, Iatrogenic | Chronic meningitis, encephalitis, human immuno-deficiency, syphilis, medications, prion disease (e.g., Creutzfeldt-Jakob disease) |
Metabolic | Thyroid disease (hypo- and hyperthyroidism), hyperammonemia |
Environmental | Carbon monoxide and heavy metal (e.g., lead, mercury) poisoning |
Supportive care and a supportive environment are as important in the management of Alzheimer’s disease as they are with delirium (see above). To date, disease-modifying treatment (agents that reverse the course of illness) for Alzheimer’s disease remains elusive, but intense research continues. 26
Symptomatic therapy for AD includes the cholinesterase inhibitors (ChE-I, e.g., donepezil, rivastigmine, galantamine), which act by increasing brain levels of ACh, and the nonselective N-methyl-D-aspartate (NMDA) glutamate receptor blocker memantine. 27 The value of these cognitive enhancers is established in patients with mild to moderate AD, 28 but their role in advanced dementia and in MCI 29 is not established. Antipsychotics and sedatives are best avoided and should be reserved for situations when the main goal of treatment is to prevent personal physical harm and harm to others (see delirium above).
Complications
Alzheimer’s disease and other dementias predispose the patient to several complications, including injury and trauma (body and head), malnutrition, incontinence of urine and stools, aspiration and aspiration pneumonia related to dysphagia, and pressure ulcers secondary to reduced mobility or immobility. In later stages of the disease, some patients develop seizures.
17.5.2 Stroke: Focus on Ischemic Stroke
Stroke is the fourth most common cause of death (a stroke-related death occurs every 4 minutes) in the U.S., it afflicts 750,000 to 800,000 people annually, and 25% of all strokes occur in individuals with a prior cerebral ischemic event. Also, stroke is a leading cause of long-term disability in the U.S., and the estimated annual cost of stroke approaches $40 billion. 30
Causes and Risk Factors
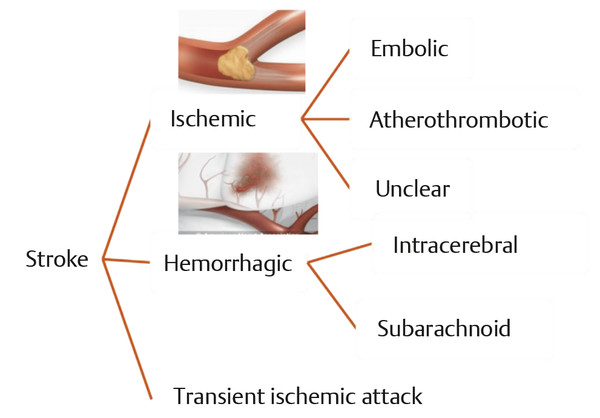
There are two major types of stroke ( ▶ Fig. 17.12): ischemic stroke and hemorrhagic stroke. 31 Hemorrhagic strokes comprise approximately 15 to 20% of all strokes and are caused by blood bursting into the substance of the brain (intracerebral hemorrhage, ICH), into the spinal fluid cavities of the brain, the ventricles (intraventricular hemorrhage), or between the layers that cushion the brain, the meninges (subarachnoid hemorrhage, SAH). Patients with SAH or ICH are more likely to be severely disabled or to die from stroke than those afflicted by cerebral infarct.
Fig. 17.12 Types of stroke.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


