Fig. 3.1
Systemic bacterial sepsis with peripheral shut-down and digital ischaemia and necrosis
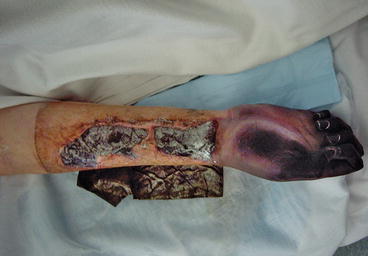
Fig. 3.2
Systemic bacterial sepsis with more advanced peripheral shut-down with ischaemia and necrosis of the hand and digits (same patient as 3.1)
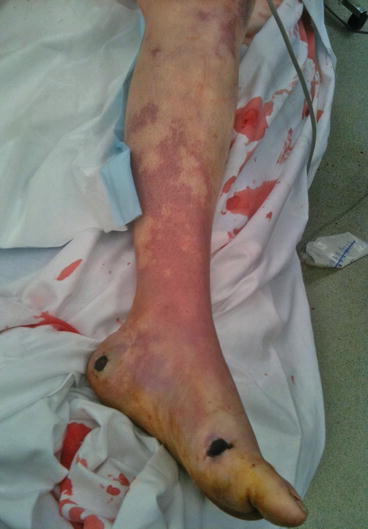
Fig. 3.3
Systemic bacterial sepsis with mottling from peripheral shut-down (same patient as 3.1)
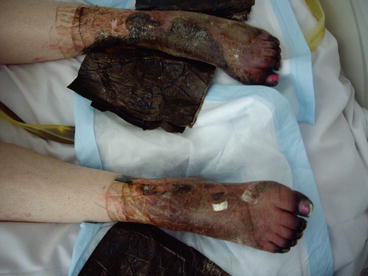
Fig. 3.4
Systemic bacterial sepsis with more advanced peripheral shut-down with ischaemia and necrosis of the feet and legs (same patient as 3.1)
Surgical Site Infections: Frequency and Impact
SSIs (also called surgical wound infections, SWIs) are the second or third most common type of healthcare-acquired infections and affect surgery worldwide, with considerable social, professional, legal, and economic impact (Mangram et al. 1999). SSIs were listed as the second most common adverse event among hospitalized patients in a 1984 study in hospitals in New York State (Brennan et al. 1991; Leape et al. 1991). The US Centers for Disease Control (CDC) Guidelines for Prevention of Surgical Site Infection1 reported an overall rate of 2.6 % SSIs among approximately 600,000 operations in US hospitals from an analysis within a study period between 1986 and 1996. It has been estimated that on average, each SSI increases the stay in hospital by about 7–10 days and results in about US$2,000 to 3,000 of extra costs (Mangram et al. 1999). Given an annual number of about 30 million operations in the US, this results in more than 700,000 SSIs annually and more than US$2 billion of extra healthcare costs. In Australia, the overall infection rate was estimated at 4.6 % in a national study published in 1988 (McLaws et al. 1988) and ranges between about 1.2 and 7.5 %, depending on the type of operation, in the reports of the Victorian Nosocomial Infection Surveillance System (http://www.vicniss.org.au). According to the Australian Institute of Health and Welfare, the estimated annual excess healthcare cost in Australia from SSIs is about A$268 million (Australian Council for Safety and Quality in Health Care 2004). European data have been found to vary widely, with SSI rates reported between 1.5 and 20 % and the annual economic burden in the range of Eur 1.47 to 19.1 billion (Leaper et al. 2004).
Causes and Risk Factors of Surgical Site Infections
The causation of SSIs is complex and multifactorial, with many contributing and preventing factors involved (Mangram et al. 1999). Thus, it becomes very difficult, often impossible, to determine the “cause” in a given case of SSI. In a general sense, the risk of SSI is determined by the “dose” of microbial contamination to the surgical site, the virulence of the pathogen, and the resistance (or lack thereof) of the patient (Mangram et al. 1999). The latter includes underlying illnesses, immunological disturbances, and the type and site of surgery. Various risk factors for SSIs can be broadly divided into host factors, preoperative factors, surgical factors, and environmental factors (Table 3.1).
Table 3.1
Risk factors for surgical site infections (SSI)
Host (patient) factors |
Old age |
Severe underlying illness |
Obesity or malnutrition |
Diabetes mellitus |
Smoking |
Compromised immunity |
Anemia and hypoxic states |
Presence of other infections |
Skin diseases |
Bleeding and hematoma formation |
Wound contamination |
Foreign material, implants |
Devitalized, tissue injury, ischemic tissues |
Tension, technical factors |
Prolonged postoperative hospital stay |
Postoperative contamination, poor wound care |
Preoperative factors |
Prolonged preoperative hospital stay |
Inappropriate hair removal (e.g., early shaving) |
Inadequate antimicrobial prophylaxis |
Carriage of Staphylococcus aureus, particularly if methicillin resistant (MRSA) |
Surgical factors |
Inadequate skin antisepsis |
Inadequate surgical hand antisepsis (scrub) |
Emergency procedure |
High surgical workload |
Infrequent procedure performed at an institution |
Lack of experience, poor surgical technique/ sterility |
Prosthetic implants |
Prolonged operation time |
Inadequate use of wound drains |
Lack of SSI surveillance |
Environmental and health system factors |
Inadequately sterilized surgical items |
Inadequate operating theatre attire |
Inadequate operating theatre ventilation |
Excessive human activity during operation |
Lack of cleanliness in the operating theatre |
Overcrowding in postoperative setting |
Inadequate aftercare and nursing |
Insufficient funding and standards |
Lack of infection control monitoring and standards |
Inappropriate institutional preventive “culture” |
Insufficient surgical backup and intervention |
Poor systems and administration |
A reflection of host factors associated with SSI risk is the general preoperative physical status of a patient; this is addressed in the physical status classification of the American Society of Anesthesiologists, also called ASA score (Mangram et al. 1999). This classification divides the preoperative status of a patient into five simple, intuitive categories from normal and healthy to moribund (Table 3.2).
Table 3.2
Physical status classification, American Society of Anesthesiologists (ASA score)
Code | Patient’s preoperative physical status |
|---|---|
1 | A normal healthy patient |
2 | A patient with mild systemic disease |
3 | A patient with severe systemic disease |
4 | A patient with severe systemic disease that is a constant threat to life |
5 | A moribund patient who is not expected to survive without the operation |
6 | A declared brain-dead patient whose organs are being removed for donor purposes |
Most SSIs are caused by microorganisms from patients’ skin, mucous membranes, and intestinal organs (Mangram et al. 1999). The role of skin microorganisms in SSI pathogenesis has been documented since the early 1900s, thus explaining the role of and providing a strong microbiological and theoretical rationale for preoperative skin antisepsis (“skin prep”) (Gröschel and Pruett 1991). Indeed, in “clean” surgery, where no mucous membranes or viscera are involved, skin flora causes most SSIs. However, in surgery through mucous membranes, intestinal surgery, or contaminated or infected surgery, the relative role of skin-based organisms decreases and that of the other sources increases, which underscores the multifactorial nature of SSIs (Gröschel and Pruett 1991; Wong 2004).
Dependent on the burden of microorganisms encountered during an operation, surgical wounds can be classified into four broad categories, ranging from clean to dirty infected (Table 3.3). These four categories are strongly linked with SSI risk and are commonly used to stratify and compare SSI rates in SSI surveillance projects (Mangram et al. 1999). Interestingly, the greatest relative improvements in lowering SSI rates in the last 20–30 years have been made in contaminated and dirty-infected surgery; the rates in clean and clean-contaminated surgery have remained relatively stable (Mangram et al. 1999). This is likely reflecting the fact that aseptic techniques have already been introduced (and must have been effective) in the first half of the twentieth century, while routine antimicrobial prophylaxis was introduced in the 1970s.
Table 3.3
Surgical wound classification
Class | Surgical procedure performed |
|---|---|
I Clean | Uninfected wound; gastrointestinal tract and other body cavities not entered; no overt contamination; wound primarily closed (e.g., skin biopsy, hernia repair); incidence of infection <2 % |
II Clean-contaminated | Respiratory, gastrointestinal, genital, or urinary tract entered under controlled conditions, but without spillage of contents or unusual contamination (e.g., small bowel resection); incidence of infection <10 % |
III Contaminated | Fresh, traumatic wounds; spillage from gastrointestinal tract; acute, non-purulent inflammation (e.g., right hemicolectomy for obstruction), including recent traumatic wounds <6 h old; incidence of infection 15–20 % |
IV Dirty infected | Gross peritoneal soiling or contamination with feces or infected material; perforated intestines; or established infection or inflammation, including traumatic wounds >6 h old or with devitalized tissue; incidence of infection >40 % |
The relative risk of development of an infection after surgery is associated with the type of wound, degree of bacterial contamination, and the time since surgery/injury. Wounds have been classified into four main classes, which is a widely used system for monitoring and communication of results between institutions. These categories are: Clean, Clean-contaminated, Contaminated and Dirty (Table 3.3).
Many different bacterial and fungal pathogens can cause SSIs, but there is a range of common pathogens. For example, S. aureus (including methicillin-resistant S. aureus, MRSA), coagulase-negative staphylococci, Enterococcus spp., Escherichia coli, Pseudomonas aeruginosa, and Enterobacter spp. are common (Mangram et al. 1999). Also, the spectrum of pathogens varies with body site, type of operation, and surgical discipline. For example, in cardiac, orthopedic, vascular, and neurosurgery, S. aureus and coagulase-negative staphylococci are more common. In gastrointestinal surgery, Gram-negative (“enteric”) bacteria and anaerobes predominate. In obstetric and gynecologic surgery, Gram-negatives, anaerobes, group B streptococci (Streptococcus agalactiae), and enterococci are more common.
Types and Diagnosis of Surgical Site Infections
According to the CDC guidelines (Mangram et al. 1999), there are three types of SSIs, depending on anatomical location:
Superficial incisional SSIs
Deep incisional SSIs
Organ/space SSIs
Superficial incisional SSIs involve skin and subcutaneous tissue; deep incisional ones involve deep soft tissue, such as fascia and muscle; and organ/space SSIs mostly involve deep-seated organs that were targets of the operation, such as the intestines, liver, and heart. Organ/space SSIs account for approximately one-third of the total number of SSIs, and by their nature, they are the most severe type, often with serious life-threatening consequences.
SSIs can be diagnosed by a combination of clinical and microbiological findings. In general, the diagnosis of an SSI can be based on the following:
Pain, localized swelling, redness, heat, and with or without fever
Purulent drainage from the wound, with or without wound dehiscence
The isolation of pathogenic microorganisms from aseptically obtained fluid or tissue
The CDC guidelines (Mangram et al. 1999) further specify a 30-day follow-up period after most common types of surgery, and a 1-year period if a surgical implant has been left in place, such as an artificial joint, prosthetic heart valve, or nonhuman vascular graft. This has important implications, because (1) SSIs may be overlooked or underdiagnosed if patients are not followed up for the appropriate period, (2) SSIs continue to cause healthcare costs after hospital discharge (Graves et al. 2006) (and even after 30 days), and (3) this requires additional logistic and personnel resources for the implementation of SSI surveillance programs.
Deep organ/space infections are often more difficult to diagnose than suggested by the above criteria. They may require radiologic examination (e.g., ultrasound, computed tomography, magnetic resonance imaging), imaging-guided needle aspiration, biopsy with histopathology, or reoperation to be diagnosed. Regardless of SSI type, it is important to note that tissue, fluid or pus samples are always preferable to swabs when samples are obtained for microbiological examination during invasive procedures, such as invasive biopsies or reoperation. Such samples have greater diagnostic yield in the microbiology laboratory than swabs, and samples are often difficult to re-obtain invasively when pathogen isolation fails.
Prevention of Surgical Site Infections
Consistent with the multifactorial nature of SSIs, there are many different measures to implement and observe in order to minimize the occurrence of SSIs. Many of these measures have developed historically, since the original implementation of antisepsis in surgery by Lister in the 1860s and then the introduction of aseptic surgery in the early 1900s (Gröschel and Pruett 1991). Thus, the contemporary practices in surgery are based on a large, complex framework of historical developments, empirical observations, many individual surgeons’ clinical experiences, microbiological and other experimental data, animal experiments, inference from knowledge of how infections are transmitted, and more recently, data from controlled clinical trials. Key measures of good surgical practice in order to prevent infections are listed in Table 3.4, and some of these measures will be explained further.
Table 3.4
Key measures of surgical practice to prevent infections
Measures of asepsis and antisepsis |
Implement and observe rigorous procedures for preoperative patient skin antisepsis, including (a) choice of antiseptic with good antimicrobial kill, (b) repeated application (e.g., 3 times), and (c) sufficient contact time (e.g., 5 min) on skin before commencing operation |
Observe rigorous procedures for surgical hand/arm antisepsis (“surgical scrub”), including (a) appropriate antiseptic and (b) appropriate time for antisepsis (e.g., 3–5 min) |
Ensure that state-of-the-art sterilization facilities and procedures are in place, including process monitoring and quality controls |
Preoperative patient preparation |
Treat comorbidities if possible; optimize patient’s physical status before operation |
If possible, treat and cure preexisting infections |
Recommend smoking cessation before elective operations |
Consider screening for S. aureus carriage and decolonization with mupirocin nasal ointment and antiseptic body washes before critical elective operations |
If possible, no hair removal; if necessary, clipping preferable to shaving, to be done immediately before operation |
Preoperative antiseptic body wash on the night before, e.g., with chlorhexidine (controversial) |
Antimicrobial prophylaxis for selected operations; usually one single dose; timing 30–60 min before incision |
If possible, keep preoperative hospital stay short |
Surgical personnel factors |
Good personal hygiene |
Appropriate operating theatre attire and personal protective devices, including (a) scrub suits and shoes dedicated only for theatre area, (b) caps/hats and face masks for inside theatre, (c) additional sterile gowns and gloves during operation |
Do not wear jewelry or artificial fingernails |
Observe “sterile” gowning and gloving techniques |
Maintain active vigilance against contamination and breaches of aseptic techniques |
Provide leadership and constructive criticism, if applicable, and positive example for other staff |
Environmental factors |
Use positive pressure-ventilated operating theatre suites, preferably with HEPA (high-efficiency particulate air) filtering and laminar airflow, with 20 or more air exchanges per hour |
Clean and/or disinfect operating theatre surfaces between each case and after any spills |
Keep high level of general cleanliness and hygiene in the operating theatre |
Restrict personnel movement through doors to a minimum |
Disinfect or replace anesthetic equipment between cases |
Use ultraclean environment for joint replacement surgery |
Intraoperative factors |
Observe “sterile” techniques and respect “sterile” areas within operating theatre |
Use double gloving or re-gloving in prolonged or joint surgery |
Use sterile drapes and covers and create an appropriate operative field |
Redrape areas or re-gown personnel after wetting, spills, or contamination |
Strictly observe rules of maintaining and respecting the “sterile field” during operation |
Use good hemostasis and gentle tissue handling, minimize tension |
Avoid spills from the intestines or other nonsterile body sites |
Maintain good oxygenation and blood circulation during anesthesia |
Remove devitalized, ischemic, or infected tissues or collections |
Check and maintain normal blood glucose levels, even in nondiabetic patients |
Avoid blood transfusion unless essential |
Maintain normal patient body temperature (normothermia), e.g., by heated blankets |
Use wound drains sparingly |
Postoperative factors |
Protect surgical site with sterile dressing for ~48 h, keep clean and dry |
Replace dirty or contaminated dressings using sterile techniques |
Educate patient in good wound care |
SSI surveillance |
Implement a surveillance program for SSIs |
Use CDC classifications for SSIs (Mangram et al. 1999) |
Use inpatient and outpatient case-finding methods to detect infection |
Educate patients in early detection/reporting of wound complications |
Use suitable surgeon monitoring and confidential feedback systems |
Compare data within and between institutions |
Utilize SSI surveillance data for surgical practice improvement |
Measures of Asepsis and Antisepsis
Measures of antisepsis, disinfection, and sterilization are essential components of modern surgery; they allowed the rapid developments in surgery that began in the early twentieth century (Gröschel and Pruett 1991). Sterilization is essential for the usage of surgical instruments and other supplies needed during operations. Main sterilization techniques are:
Autoclaving for heat- and moisture-stable items
Dry heat sterilization for other heat-stable items
Ethylene oxide or hydrogen peroxide plasma sterilization for heat-sensitive items
Most major hospitals have sterilization departments, and it is important that surgical supplies (e.g., instruments) are cleaned well before re-sterilization and that all sterilization processes are monitored and quality controlled. Surgeons are now rarely confronted with technical questions of sterilization, and failure of sterilization processes is rare at facilities where a good infrastructure is in place, but when failure occurs, it can have severe consequences for patient safety.
There are two major types of antisepsis involved in surgical procedures:
Surgical hand and arm antisepsis (“surgical scrub”)
Preoperative skin antisepsis on the patient (“skin prep”)
The rationale for surgical hand antisepsis is to significantly reduce the bacterial count on arm and hand skin to reduce the transfer of microorganisms to the patient by (1) unrecognized punctures in surgical gloves during the operation (up to ~35 %), (2) tiny holes present in a small percentage of new gloves, and perhaps (3) accidental touching of the wound after removal of gloves (World Health Organization 2009). This rationale is further supported by published clusters of SSI cases following breaches of hand antisepsis protocols (World Health Organization 2009; Grinbaum et al. 1995). The protocols and substances vary; for example, continental European countries have traditionally preferred alcohol solutions, while aqueous detergent-based antisepsis with chlorhexidine or povidone-iodine has been preferred in the USA, UK, and Australasia. Time of surgical hand antisepsis is another parameter; typically, 3–5 min (up to 10) are recommended. Commonly, alcohol-based protocols lead to a reduction of transient and resident microorganisms by about 2–3 logs (~100–1,000-fold), while detergent-based methods reach about 1- to 2-log reductions (Gröschel and Pruett 1991). While no differences in SSI rates have been detected so far, other advantages of alcohols are better skin tolerability of commercial products with added emollients (i.e., skin moisturizers) and shorter overall scrubbing times. There is some evidence that after the initial scrub for the day, subsequent scrubs can be reduced to 1 min with equivalently effective antisepsis, especially for chlorhexidine-based wash solutions (Watts et al. 1981).
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


