Duration of prophylaxis. New recommendations for a shortened postoperative course of antimicrobials involving a single dose or continuation for less than 24 hours are provided. Further clarity on the lack of need for postoperative antimicrobial prophylaxis based on the presence of indwelling drains and intravascular catheters is included.
Common principles. A section addressing concepts that apply to all types of surgical procedures has been added. Expanded and new recommendations are provided for plastic, urology, cardiac, and thoracic procedures, as well as clarity on prophylaxis when implantable devices are inserted. The latest information on the use of mupirocin and on the role of vancomycin in surgical prophylaxis is summarized in these updated guidelines.
Application of Guidelines to Clinical Practice. Recommendations are provided for adult (age 19 years or older) and pediatric (age 1–18 years) patients. These guidelines do not specifically address newborn (premature and full-term) infants. While the guidelines do not address all concerns for patients with renal or hepatic dysfunction, antimicrobial prophylaxis often does not need to be modified for these patients when given as a single preoperative dose before surgical incision.
The recommendations herein may not be appropriate for use in all clinical situations. Decisions to follow these recommendations must be based on the judgment of the clinician and consideration of individual patient circumstances and available resources.
These guidelines reflect current knowledge of antimicrobial prophylaxis in surgery. Given the dynamic nature of scientific information and technology, periodic review, updating, and revisions are to be expected.
Special Patient Populations. Pediatric patients. Pediatric patients undergo a number of procedures similar to adults that may warrant antimicrobial prophylaxis. Although pediatric-specific prophylaxis data are sparse, available data have been evaluated and are presented in some of the procedure-specific sections of these guidelines. Selection of antimicrobial prophylactic agents mirrors that in adult guidelines, with the agents of choice being first- and second-generation cephalosporins, reserving the use of vancomycin for patients with documented β-lactam allergies.19,20 While the use of a penicillin with a β-lactamase inhibitor in combination with cefazolin or vancomycin and gentamicin has also been studied in pediatric patients, the number of patients included in these evaluations remains small.20–23 As with adults, there is little evidence supporting the use of vancomycin, alone or in combination with other antimicrobials, for routine perioperative antimicrobial prophylaxis in institutions that have a high prevalence of methicillin-resistant Staphylococcus aureus (MRSA). Vancomycin may be considered in children known to be colonized with MRSA and, in one retrospective historical cohort study, was shown to decrease MRSA infections.21 Mupirocin use has been studied in and is efficacious in children colonized with MRSA, but there are limited data supporting its use perioperatively.24–30 However, there is little reason to think that the impact and effect would be any different in children, so its use may be justified. Additional studies in this setting are needed to establish firm guidelines.
Unless noted in specific sections, all recommendations for adults are the same for pediatric patients, except for dosing. In most cases, the data in pediatric patients are limited and have been extrapolated from adult data; therefore, nearly all pediatric recommendations are based on expert opinion. In some sections, pediatric efficacy data do not exist and thus are not addressed in these guidelines. Fluoroquinolones should not be routinely used for surgical prophylaxis in pediatric patients because of the potential for toxicity in this population. The same principle of preoperative dosing within 60 minutes before incision has been applied to pediatric patients.20–23 Additional intraoperative dosing may be needed if the duration of the procedure exceeds two half-lives of the antimicrobial agent or there is excessive blood loss during the procedure.19,21 As with adult patients, single-dose prophylaxis is usually sufficient. If antimicrobial prophylaxis is continued postoperatively, the duration should be less than 24 hours, regardless of the presence of intravascular catheters or indwelling drains.19,22,23,31,32 There are sufficient pharmacokinetic studies of most agents to recommend pediatric dosages that provide adequate systemic exposure and, presumably, efficacy comparable to that demonstrated in adults. Therefore, the pediatric dosages provided in these guidelines are based largely on pharmacokinetic data and the extrapolation of adult efficacy data to pediatric patients. Because few clinical trials have been conducted in pediatric surgical patients, strength of evidence criteria have not been applied to these recommendations. With few exceptions (e.g., aminoglycoside dosages), pediatric dosages should not exceed the maximum adult recommended dosages. Generally, if dosages are calculated on a milligram-per-kilogram basis for children weighing more than 40 kg, the calculated dosage will exceed the maximum recommended dosage for adults; adult dosages should therefore be used.
Patients with prosthetic implants. For patients with existing prosthetic implants who undergo an invasive procedure, there is no evidence that antimicrobial prophylaxis prevents infections of the implant. However, updated guidelines from the American Heart Association (AHA) suggest that prophylaxis may be justified in a limited subset of patients for the prevention of endocarditis.11
Common Principles and Procedure-Specific Guidelines. The Common Principles section has been developed to provide information common to many surgical procedures. These principles are general recommendations based on currently available data at the time of publication that may change over time; therefore, these principles need to be applied with careful attention to each clinical situation. Detailed information pertinent to specific surgical procedures is included in the procedure-specific sections of these guidelines.
In addition to patient- and procedure-specific considerations, several institution-specific factors must be considered by practitioners before instituting these guidelines. The availability of antimicrobial agents at the institution may be restricted by local antimicrobial-use policy or lack of approval for use by regulatory authorities. Medications that are no longer available or not approved for use by the Food and Drug Administration (FDA) are so noted. Local resistance patterns should also be considered in selecting antimicrobial agents and are discussed in the colonization and resistance patterns section of the Common Principles section.
Requirements for Effective Surgical Prophylaxis
Appendix A lists the wound classification criteria currently used by the CDC National Healthcare Safety Network (NHSN) and Healthcare Infection Control Practices Advisory Committee (HICPAC).33–35
Criteria for defining an SSI have also been established by NHSN (Appendix B).8,36 These definitions assist in evaluating the importance of providing antimicrobial prophylaxis and the potential consequences of infection, including the need for treatment. Some criteria vary slightly by procedure.
Although antimicrobial prophylaxis plays an important role in reducing the rate of SSIs, other factors such as attention to basic infection-control strategies,37 the surgeon’s experience and technique, the duration of the procedure, hospital and operating-room environments, instrument-sterilization issues, preoperative preparation (e.g., surgical scrub, skin antisepsis, appropriate hair removal), perioperative management (temperature and glycemic control), and the underlying medical condition of the patient may have a strong impact on SSI rates.5,8 These guidelines recognize the importance of these other factors but do not include a discussion of or any recommendations regarding these issues beyond the optimal use of prophylactic antimicrobial agents. Patient-related factors associated with an increased risk of SSI include extremes of age, nutritional status, obesity, diabetes mellitus, tobacco use, coexistent remote body-site infections, altered immune response, corticosteroid therapy, recent surgical procedure, length of preoperative hospitalization, and colonization with microorganisms. Antimicrobial prophylaxis may be justified for any procedure if the patient has an underlying medical condition associated with a high risk of SSI or if the patient is immunocompromised (e.g., malnourished, neutropenic, receiving immunosuppressive agents).
Antimicrobial prophylaxis may be beneficial in surgical procedures associated with a high rate of infection (i.e., clean-contaminated or contaminated procedures) and in certain clean procedures where there are severe consequences of infection (e.g., prosthetic implants), even if infection is unlikely. While prophylactic antimicrobials are not indicated for some clean surgical procedures,8 available data suggest that the relative risk reduction of SSI from the use of antimicrobial prophylaxis is the same in clean and in higher-risk procedures.38 The decision to use prophylaxis depends on the cost of treating and the morbidity associated with infection compared with the cost and morbidity associated with using prophylaxis. Antimicrobial prophylaxis is justified for most clean-contaminated procedures. The use of antimicrobial agents for dirty procedures (Appendix A) or established infections is classified as treatment of presumed infection, not prophylaxis. See the procedure-specific sections for detailed recommendations.
Quality-Improvement Efforts. National, state, local, and institutional groups have developed and implemented collaborative efforts to improve the appropriateness of surgical antimicrobial prophylaxis. Various process and outcomes measures are employed, and results are disseminated. Institutional epidemiology and infection-control programs, state-based quality-improvement campaigns (e.g., the Michigan Surgical Quality Collaborative, the Washington State Surgical Clinical Outcomes Assessment Program39,40), CDC, NHSN, the National Surgical Quality Improvement Program, the Joint Commission, and the National Quality Forum have been instrumental in developing programs to prevent SSIs.
Over the past decade or more, several organizations, payers, and government agencies, including the Centers for Medicare and Medicaid Services (CMS), have established national quality-improvement initiatives to further improve the safety and outcomes of health care, including surgery.41–47 One area of focus in these initiatives for patients undergoing surgical procedures is the prevention of SSIs. The performance measures used, data collection and reporting requirements, and financial implications vary among the initiatives. The Surgical Care Improvement Project (SCIP) began in 2002 as the Surgical Infection Prevention (SIP) project, focusing on the timing, selection, and duration of prophylactic antimicrobial agents.41,42 The SIP project was expanded to SCIP to include additional process measures surrounding patient safety and care during surgical procedures, including glucose control, venous thromboembolism prophylaxis, hair removal, and temperature control. Similar measures have been adopted by the Joint Commission.43 The Physicians Quality Reporting System was established in 2006 to provide financial incentives to physicians meeting performance standards for quality measures, including surgery-related measures similar to those reported for SCIP and the Joint Commission.44 Data are required to be collected by institutions and reported to payers.42,44,46 Data for CMS and the Physicians Quality Reporting System measures are displayed on public websites to allow consumers to compare performance among hospitals. Institutional data collection and reporting are required, with financial incentives tied to performance to varying degrees, including payment for reporting, payment increases for meeting or exceeding minimum levels of performance, payment reduction for poor performance, and lack of payment for the development of surgical complications, such as mediastinitis.
Quality-improvement initiatives and mandated performance reporting are subject to change, so readers of these guidelines are advised to consult their local or institutional quality-improvement departments for new developments in requirements for measures and data reporting that apply to their practice.
Cost Containment. Few pharmacoeconomic studies have addresed surgical antimicrobial prophylaxis; therefore, a cost-minimization approach was employed in developing these guidelines. The antimicrobial agent recommendations are based primarily on efficacy and safety. Individual institutions must consider their acquisition costs when implementing these guidelines.
Additional cost savings may be realized through collaborative management by pharmacists and surgeons to select the most cost-effective agent and minimize or eliminate postoperative dosing.48–50 The use of standardized antimicrobial order sets, automatic stop-order programs, and educational initiatives has been shown to facilitate the adoption of guidelines for surgical antimicrobial prophylaxis.51–58
Common Principles
Ideally, an antimicrobial agent for surgical prophylaxis should (1) prevent SSI, (2) prevent SSI-related morbidity and mortality, (3) reduce the duration and cost of health care (when the costs associated with the management of SSI are considered, the cost-effectiveness of prophylaxis becomes evident),51,52 (4) produce no adverse effects, and (5) have no adverse consequences for the microbial flora of the patient or the hospital.53 To achieve these goals, an antimicrobial agent should be (1) active against the pathogens most likely to contaminate the surgical site, (2) given in an appropriate dosage and at a time that ensures adequate serum and tissue concentrations during the period of potential contamination, (3) safe, and (4) administered for the shortest effective period to minimize adverse effects, the development of resistance, and costs.8,59,60
The selection of an appropriate antimicrobial agent for a specific patient should take into account the characteristics of the ideal agent, the comparative efficacy of the antimicrobial agent for the procedure, the safety profile, and the patient’s medication allergies. A full discussion of the safety profile, including adverse events, drug interactions, contraindications, and warnings, for each antimicrobial agent is beyond the scope of these guidelines. Readers of these guidelines should review the FDA-approved prescribing information and published data for specific antimicrobial agents before use. For most procedures, cefazolin is the drug of choice for prophylaxis because it is the most widely studied antimicrobial agent, with proven efficacy. It has a desirable duration of action, spectrum of activity against organisms commonly encountered in surgery, reasonable safety, and low cost. There is little evidence to suggest that broad-spectrum antimicrobial agents (i.e., agents with broad in vitro antibacterial activity) result in lower rates of postoperative SSI compared with older antimicrobial agents with a narrower spectrum of activity. However, comparative studies are limited by small sample sizes, resulting in difficulty detecting a significant difference between antimicrobial agents; therefore, antimicrobial selection is based on cost, safety profile, ease of administration, pharmacokinetic profile, and bactericidal activity.
Common Surgical Pathogens
The agent chosen should have activity against the most common surgical-site pathogens. The predominant organisms causing SSIs after clean procedures are skin flora, including S. aureus and coagulase-negative staphylococci (e.g., Staphylococcus epidermidis).61 In clean-contaminated procedures, including abdominal procedures and heart, kidney, and liver transplantations, the predominant organisms include gram-negative rods and enterococci in addition to skin flora. Additional details on common organisms can be found in procedure-specific sections of these guidelines.
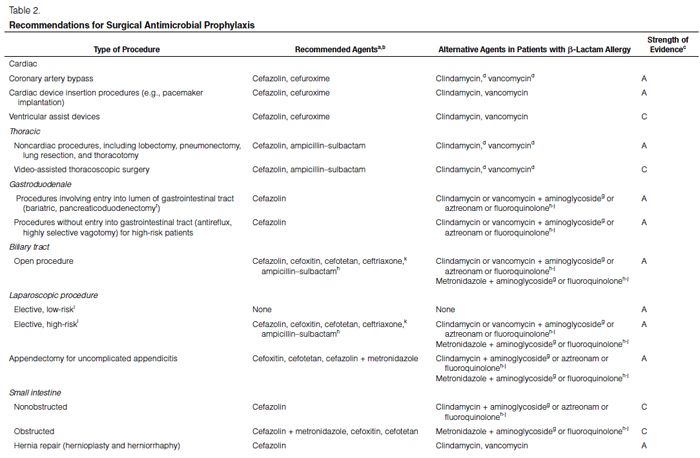
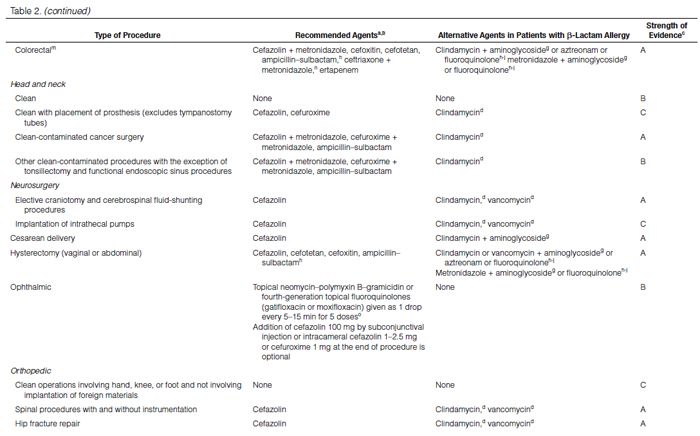
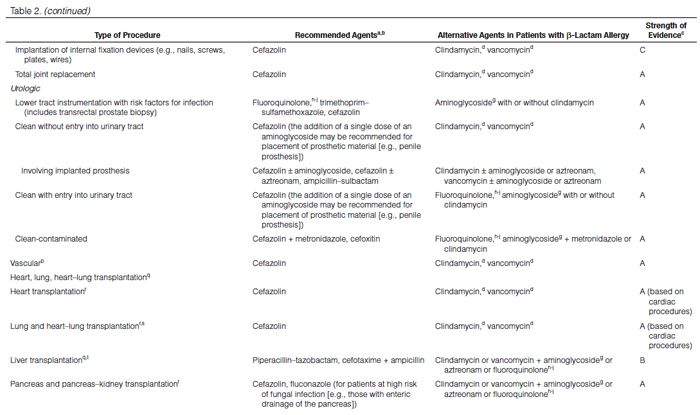
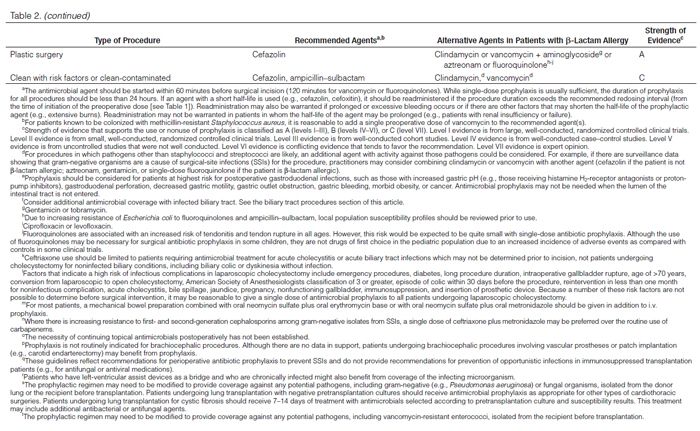
Recommendations for the selection of prophylactic antimicrobials for various surgical procedures are provided in Table 2. Adult and pediatric dosages are included in Table 1. Agents that are FDA-approved for use in surgical antimicrobial prophylaxis include cefazolin, cefuroxime, cefoxitin, cefotetan, ertapenem, and vancomycin.62–67
Trends in Microbiology. The causative pathogens associated with SSIs in U.S. hospitals have changed over the past two decades. Analysis of National Nosocomial Infections Surveillance (NNIS) System data found that the percentage of SSIs caused by gram-negative bacilli decreased from 56.5% in 1986 to 33.8% in 2003.68 S. aureus was the most common pathogen, causing 22.5% of SSIs during this time period. NHSN data from 2006 to 2007 revealed that the proportion of SSIs caused by S. aureus increased to 30%, with MRSA comprising 49.2% of these isolates.61 In a study of patients readmitted to U.S. hospitals between 2003 and 2007 with a culture-confirmed SSI, the proportion of infections caused by MRSA increased significantly from 16.1% to 20.6% (p < 0.0001).69 MRSA infections were associated with higher mortality rates, longer hospital stays, and higher hospital costs compared with other infections.
Spectrum of Activity. Antimicrobial agents with the narrowest spectrum of activity required for efficacy in preventing infection are recommended in these guidelines. Alternative antimicrobial agents with documented efficacy are also listed herein. Individual health systems must consider local resistance patterns of organisms and overall SSI rates at their site when adopting these recommendations. Resistance patterns from organisms causing SSIs—in some cases procedure-specific resistance patterns—should take precedence over hospitalwide antibiograms.
Vancomycin. In 1999, HICPAC, an advisory committee to CDC and the Secretary of the Department of Health and Human Services, collaborated with other major organizations to develop recommendations for preventing and controlling vancomycin resistance.70 The recommendations are echoed by these and other guidelines.6,7,41,71 Routine use of vancomycin prophylaxis is not recommended for any procedure.8 Vancomycin may be included in the regimen of choice when a cluster of MRSA cases (e.g., mediastinitis after cardiac procedures) or methicillin-resistant coagulase-negative staphylococci SSIs have been detected at an institution. Vancomycin prophylaxis should be considered for patients with known MRSA colonization or at high risk for MRSA colonization in the absence of surveillance data (e.g., patients with recent hospitalization, nursing-home residents, hemodialysis patients).5,41,72 In institutions with SSIs attributable to community-associated MRSA, antimicrobial agents with known in vitro activity against this pathogen may be considered as an alternative to vancomycin.
Each institution is encouraged to develop guidelines for the proper use of vancomycin. Although vancomycin is commonly used when the risk for MRSA is high, data suggest that vancomycin is less effective than cefazolin for preventing SSIs caused by methicillin-susceptible S. aureus (MSSA).73,74 For this reason, vancomycin is used in combination with cefazolin at some institutions with both MSSA and MRSA SSIs. For procedures in which pathogens other than staphylococci and streptococci are likely, an additional agent with activity against those pathogens should be considered. For example, if there are surveillance data showing that gram-negative organisms are a cause of SSIs for the procedure, practitioners may consider combining vancomycin with another agent (cefazolin if the patient does not have a β-lactam allergy; an aminoglycoside [gentamicin or tobramycin], aztreonam, or single-dose fluoroquinolone if the patient has a β-lactam allergy). The use of vancomycin for MRSA prophylaxis does not supplant the need for routine surgical prophylaxis appropriate for the type of procedure. When vancomycin is used, it can almost always be used as a single dose due to its long half-life.
Colonization and Resistance. A national survey determined that S. aureus nasal colonization in the general population decreased from 32.4% in 2001–02 to 28.6% in 2003–04 (p < 0.01), whereas the prevalence of colonization with MRSA increased from 0.8% to 1.5% (p < 0.05) during the same time periods.75 Colonization with MRSA was independently associated with health care exposure among men, having been born in the United States, age of >60 years, diabetes, and poverty among women. Similarly, children are colonized with S. aureus and MRSA, but colonization varies by age. Children under 5 years of age have the highest rates, mirroring rates seen in patients over age 60 years.76 The rates drop in children between 5 and 14 years of age and gradually increase to rates seen in the adult population. Lo et al.77 reported that in a large cohort of children, 28.1% were colonized with S. aureus between 2004 and 2006. Between 2007 and 2009, 23.3% of children were colonized with S. aureus, but the proportion of children colonized with MRSA had increased from 8.1% in 2004 to 15.1% in 2009.
Surgical antimicrobial prophylaxis can alter individual and institutional bacterial flora, leading to changes in colonization rates and increased bacterial resistance.78–84 Surgical prophylaxis can also predispose patients to Clostridium difficile-associated colitis.81 Risk factors for development of C. difficile-associated colitis include longer duration of prophylaxis or therapy and use of multiple antimicrobial agents.85 Limiting the duration of antimicrobial prophylaxis to a single preoperative dose can reduce the risk of C. difficile disease.
The question of what antimicrobial surgical prophylaxis to use for patients known to be colonized or recently infected with multidrug-resistant pathogens cannot be answered easily or in a manner that can be applied uniformly to all patient scenarios. Whether prophylaxis should be expanded to provide coverage for these pathogens depends on many factors, including the pathogen, its antimicrobial susceptibility profile, the host, the procedure to be performed, and the proximity of the likely reservoir of the pathogen to the incision and operative sites. While there is no evidence on the management of surgical antimicrobial prophylaxis in a patient with past infection or colonization with a resistant gram-negative pathogen, it is logical to provide prophylaxis with an agent active against MRSA for any patient known to be colonized with this gram-positive pathogen who will have a skin incision; specific prophylaxis for a resistant gram-negative pathogen in a patient with past infection or colonization with such a pathogen may not be necessary for a purely cutaneous procedure. Similarly, a patient colonized with vancomycin-resistant enterococci (VRE) should receive prophylaxis effective against VRE when undergoing liver transplantation but probably not when undergoing an umbilical hernia repair without mesh placement. Thus, patients must be treated on a case-by-case basis, taking into account multiple considerations.
Patients receiving therapeutic antimicrobials for a remote infection before surgery should also be given antimicrobial prophylaxis before surgery to ensure adequate serum and tissue levels of antimicrobials with activity against likely pathogens for the duration of the operation. If the agents used therapeutically are appropriate for surgical prophylaxis, administering an extra dose within 60 minutes before surgical incision is sufficient. Otherwise, the antimicrobial prophylaxis recommended for the planned procedure should be used. For patients with indwelling tubes or drains, consideration may be given to using prophylactic agents active against pathogens found in these devices before the procedure, even though therapeutic treatment for pathogens in drains is not indicated at other times. For patients with chronic renal failure receiving vancomycin, a preoperative dose of cefazolin should be considered instead of an extra dose of vancomycin, particularly if the probable pathogens associated with the procedure are gram-negative. In most circumstances, elective surgery should be postponed when the patient has an infection at a remote site.
Allergy to β-Lactam Antimicrobials. Allergy to β-lactam antimicrobials may be a consideration in the selection of surgical prophylaxis. The β-lactam antimicrobials, including cephalosporins, are the mainstay of surgical antimicrobial prophylaxis and are also the most commonly implicated drugs when allergic reactions occur. Because the predominant organisms in SSIs after clean procedures are gram-positive, the inclusion of vancomycin may be appropriate for a patient with a life-threatening allergy to β-lactam antimicrobials.
Although true Type 1 (immunoglobulin E [IgE]-mediated) cross-allergic reactions between penicillins, cephalosporins, and carbapenems are uncommon, cephalosporins and carbapenems should not be used for surgical prophylaxis in patients with documented or presumed IgE-mediated penicillin allergy. Confusion about the definition of true allergy among patients and practitioners leads to recommendations for alternative antimicrobial therapy with the potential for a lack of efficacy, increased costs, and adverse events.86,87 Type 1 anaphylactic reactions to antimicrobials usually occur 30–60 minutes after administration. In patients receiving penicillins, this reaction is a life-threatening emergency that precludes subsequent use of penicillins.88 Cephalosporins and carbapenems can safely be used in patients with an allergic reaction to penicillins that is not an IgE-mediated reaction (e.g., anaphylaxis, urticaria, bronchospasm) or exfoliative dermatitis (Stevens-Johnson syndrome, toxic epidermal necrolysis), a life-threatening hypersensitivity reaction that can be caused by β-lactam antimicrobials and other medications.88,89 Patients should be carefully questioned about their history of antimicrobial allergies to determine whether a true allergy exists before selection of agents for prophylaxis. Patients with allergies to cephalosporins, penicillins, or both have been excluded from many clinical trials. Alternatives to β-lactam antimicrobials are provided in Table 2 based mainly on the antimicrobial activity profiles against predominant procedure-specific organisms and available clinical data.
The preferred route of administration varies with the type of procedure, but for a majority of procedures, i.v. administration is ideal because it produces rapid, reliable, and predictable serum and tissue concentrations.
Timing of Initial Dose. Successful prophylaxis requires the delivery of the antimicrobial to the operative site before contamination occurs. Thus, the antimicrobial agent should be administered at such a time to provide serum and tissue concentrations exceeding the minimum inhibitory concentration (MIC) for the probable organisms associated with the procedure, at the time of incision, and for the duration of the procedure.41,90 In 1985, DiPiro et al.91 demonstrated that higher serum and tissue cephalosporin concentrations at the time of surgical incision and at the end of the procedure were achieved when the drugs were given intravenously at the time of anesthesia induction compared with administration in the operating room. The average interval between antimicrobial administration and incision was 17–22 minutes91 (Dellinger EP, personal communication, 2011 May).
A prospective evaluation of 1708 surgical patients receiving antimicrobial prophylaxis found that preoperative administration of antimicrobials within 2 hours before surgical incision decreased the risk of SSI to 0.59%, compared with 3.8% for early administration (2–24 hours before surgical incision) and 3.3% for any postoperative administration (any time after incision).92 In a study of 2048 patients undergoing coronary bypass graft or valve replacement surgery receiving vancomycin prophylaxis, the rate of SSI was lowest in those patients in whom an infusion was started 16–60 minutes before surgical incision.93 This time interval (16–60 minutes before incision) was compared with four others, and the rates of SSIs were significantly lower when compared with infusions given 0–15 minutes before surgical incision (p < 0.01) and 121–180 minutes before incision (p = 0.037). The risk of infection was higher in patients receiving infusions 61–120 minutes before incision (odds ratio [OR], 2.3; 95% confidence interval [CI], 0.98–5.61) and for patients whose infusions were started more than 180 minutes before surgical incision (OR, 2.1; 95% CI, 0.82–5.62).93
In a large, prospective, multicenter study from the Trial to Reduce Antimicrobial Prophylaxis Errors (TRAPE) study group, the timing, duration, and intraoperative redosing of antimicrobial prophylaxis and risk of SSI were evaluated in 4472 patients undergoing cardiac surgery, hysterectomy, or hip or knee arthroplasty.94 The majority of patients (90%) received antimicrobial prophylaxis per the SCIP guidelines.41 Patients were assigned to one of four groups for analysis. Group 1 (n = 1844) received a cephalosporin (or other antimicrobial with a short infusion time) administered within 30 minutes before incision or vancomycin or a fluoroquinolone within one hour before incision. Group 2 (n = 1796) received a cephalosporin 31–60 minutes before incision or vancomycin 61–120 minutes before incision. Group 3 (n = 644) was given antimicrobials earlier than recommended, and group 4 (n = 188) received their initial antimicrobial doses after incision. The infection risk was lowest in group 1 (2.1%), followed by group 2 (2.4%) and group 3 (2.8%). The risk of infection was highest in group 4 (5.3%, p = 0.02 compared with group 1). When cephalosporins and other antimicrobials with short infusion times were analyzed separately (n = 3656), the infection rate with antimicrobials administered within 30 minutes before incision was 1.6% compared with 2.4% when antimicrobials were administered 31–60 minutes before incision (p = 0.13).
In a multicenter Dutch study of 1922 patients undergoing total hip arthroplasty, the lowest SSI rate was seen in patients who received the antimicrobial during the 30 minutes before incision.95 The highest risk for infection was found in patients who received prophylaxis after the incision.
It seems intuitive that the entire antimicrobial dose should be infused before a tourniquet is inflated or before any other procedure that restricts blood flow to the surgical site is initiated; however, a study of total knee arthroplasties compared cefuroxime given 10–30 minutes before tourniquet inflation with cefuroxime given 10 minutes before tourniquet deflation and found no significant difference in SSI rates between the two groups.96
Overall, administration of the first dose of antimicrobial beginning within 60 minutes before surgical incision is recommended.41,94,97 Administration of vancomycin and fluoroquinolones should begin within 120 minutes before surgical incision because of the prolonged infusion times required for these drugs. Because these drugs have long half-lives, this early administration should not compromise serum levels of these agents during most surgical procedures. Although the recent data summarized above suggest lower infection risk with antimicrobial administration beginning within 30 minutes before surgical incision, these data are not sufficiently robust to recommend narrowing the optimal window to begin infusion to 1–30 minutes before surgical incision. However, these data do suggest that antimicrobials can be administered too close to the time of incision. Although a few articles have suggested increased infection risk with administration too close to the time of incision,93,96,97 the data presented are not convincing. In fact, all of these articles confirm the increased rate of SSI for antimicrobials given earlier than 60 minutes before incision. In one article, the infection rate for patients given an antimicrobial within 15 minutes of incision was lower than when antimicrobials were given 15–30 minutes before incision.97 In another article, small numbers of patients were reported, and an assertion of high infection rates for infusion within 15 minutes of incision was made, but no numeric data or p values were provided.98 In a third article, only 15 of over 2000 patients received antimicrobials within 15 minutes before incision.93 Earlier studies found that giving antimicrobials within 20 minutes of incision and as close as 7 minutes before incision resulted in therapeutic levels in tissue at the time of incision.41,90,91,94,97,98
Dosing. To ensure that adequate serum and tissue concentrations of antimicrobial agents for prophylaxis of SSIs are achieved, antimicrobial-specific pharmacokinetic and pharmacodynamic properties and patient factors must be considered when selecting a dose. One of the earliest controlled studies of antimicrobial prophylaxis in cardiac surgery found a lower rate of infection in patients with detectable concentrations of the drug in serum at the end of surgery compared with patients in whom the drug was undetectable.99 In another study, higher levels of antimicrobial in atrial tissue at the time of starting the pump for open-heart surgery were associated with fewer infections than were lower antimicrobial concentrations.100 In patients undergoing colectomy, infection levels were inversely related to the serum gentamicin concentration at the time of surgical closure.17 In general, it seems advisable to administer prophylactic agents in a manner that will ensure adequate levels of drug in serum and tissue for the interval during which the surgical site is open.
Weight-based dosing. The dosing of most antimicrobials in pediatric patients is based on body weight, but the dosing of many antimicrobials in adults is not based on body weight, because it is safe, effective, and convenient to use standardized doses for most of the adult patient population. Such standardized doses avoid the need for calculations and reduce the risk for medication errors. However, in obese patients, especially those who are morbidly obese, serum and tissue concentrations of some drugs may differ from those in normal-weight patients because of pharmacokinetic alterations that depend on the lipophilicity of the drug and other factors.101 Limited data are available on the optimal approach to dosing of antimicrobial agents for obese patients.102,103 If weight-based dosing is warranted for obese patients, it has not been determined whether the patient’s ideal body weight or total (i.e., actual) body weight should be used. In theory, using the ideal body weight as the basis for dosing a lipophilic drug (e.g., vancomycin) could result in subtherapeutic concentrations in serum and tissue, and the use of actual body weight for dosing a hydrophilic drug (e.g., an aminoglycoside) could result in excessive concentrations in serum and tissue. Pediatric patients weighing more than 40 kg should receive weight-based doses unless the dose or daily dose exceeds the recommended adult dose.104
Conclusive recommendations for weight-based dosing for antimicrobial prophylaxis in obese patients cannot be made because data demonstrating clinically relevant decreases in SSI rates from the use of such dosing strategies instead of standard doses in obese patients are not available in the published literature.
In a small, nonrandomized, two-phase study of morbidly obese adults undergoing gastroplasty and normal-weight adults undergoing upper abdominal surgery, blood and tissue concentrations of cefazolin after the administration of a 1-g preoperative dose were consistently lower in morbidly obese patients than in the normal-weight patients.101 The concentrations in morbidly obese patients also were lower than the MICs needed for prophylaxis against gram-positive cocci and gram-negative rods. In the second phase of the study, adequate blood and tissue cefazolin concentrations were achieved in morbidly obese patients receiving preoperative doses of cefazolin 2 g, and the rate of SSIs was significantly lower in these patients compared with morbidly obese patients receiving 1-g doses during the first phase of the study.
While the optimal cefazolin dose has not been established in obese patients, a few pharmacokinetic studies have investigated the cefazolin concentrations in serum and tissue during surgical procedures.13,105 Two small pharmacokinetic studies found that administering 1- or 2-g doses of cefazolin may not be sufficient to produce serum and tissue concentrations exceeding the MIC for the most common pathogens. In a small, single-center study, 38 adults undergoing Roux-en-Y gastric bypass surgery were classified by body mass index (BMI) in one of three groups.13 All patients were given cefazolin 2 g i.v. 30–60 minutes before the incision, followed by a second 2-g i.v. dose three hours later. The mean serum drug concentration before the second dose of cefazolin was lower than the resistance breakpoint in all three BMI groups. Serum drug concentrations were lower in patients with a high BMI than in patients with lower BMI values. Tissue drug concentrations were lower than a targeted concentration of 8 μg/mL at all measurement times, except the time of skin closure in the patients with the lowest BMIs. These results suggest that a 1-g dose of cefazolin may be inadequate for obese patients undergoing gastric bypass surgery. A weakness of the literature on drug dosing in morbidly obese patients is the practice of reporting results by BMI rather than weight.
Doubling the normal dose of cephalosporins or making fewer adjustments based on renal dysfunction may produce concentrations in obese patients similar to those achieved with standard doses in normal-weight patients.103 Considering the low cost and favorable safety profile of cefazolin, increasing the dose to 2 g for patients weighing more than 80 kg and to 3 g for those weighing over 120 kg can easily be justified.41 For simplification, some hospitals have standardized 2-g cefazolin doses for all adult patients.
Gentamicin doses have been compared for prophylaxis only in colorectal surgery, where a single dose of gentamicin 4.5 mg/kg in combination with metronidazole was more effective in SSI prevention than multiple doses of gentamicin 1.5 mg/kg every eight hours.16,17 In obese patients who weigh 20% above their ideal body weight, the dose of gentamicin should be calculated using the ideal body weight plus 40% of the difference between the actual and ideal weights.106 If gentamicin will be used in combination with a parenteral antimicrobial with activity against anaerobic agents for prophylaxis, it is probably advisable to use 4.5–5 mg/kg as a single dose.16 This dose of gentamicin has been found safe and effective in a large body of literature examining the use of single daily doses of gentamicin for therapeutic indications.106–113 When used as a single dose for prophylaxis, the risk of toxicity from gentamicin is very low.
Obese patients are often underrepresented in clinical trials and are not currently considered a special population for whom FDA requires separate pharmacokinetic studies during antimicrobial research and development by the drug manufacturer. Obesity has been recognized as a risk factor for SSI; therefore, optimal dosing of antimicrobial prophylaxis is needed in these patients.114 While a BMI of >30 kg/m2 is commonly used to define obesity, the body fat percentage (>25% in men and >31% in women) may better predict SSI risk, because the BMI may not reflect body composition. In a recent prospective cohort study of 590 patients undergoing elective surgery, there was no significant difference in SSI rates in nonobese and obese patients when the BMI was used to define obesity (12.3% versus 11.6%, respectively).115 However, when the body fat percentage (determined by bioelectrical impedance analysis) was used as the basis for identifying obesity (>25% in men and >31% in women), obese patients had a fivefold-higher risk of SSI than did nonobese patients (OR, 5.3; 95% CI, 1.2–23.1; p = 0.03). These findings suggest that body fat percentage is a more sensitive and precise measurement of SSI risk than is the BMI.
Redosing. Intraoperative redosing is needed to ensure adequate serum and tissue concentrations of the antimicrobial if the duration of the procedure exceeds two half-lives of the antimicrobial or there is excessive blood loss (i.e., >1500 mL).17,41,94,116–121 The redosing interval should be measured from the time of administration of the preoperative dose, not from the beginning of the procedure. Redosing may also be warranted if there are factors that shorten the half-life of the antimicrobial agent (e.g., extensive burns). Redosing may not be warranted in patients in whom the half-life of the antimicrobial agent is prolonged (e.g., patients with renal insufficiency or renal failure). See Table 1 for antimicrobial-specific redosing recommendations.
Duration. The shortest effective duration of antimicrobial administration for preventing SSI is not known; however, evidence is mounting that postoperative antimicrobial administration is not necessary for most procedures.6,7,41,122–124 The duration of antimicrobial prophylaxis should be less than 24 hours for most procedures. Cardiothoracic procedures for which a prophylaxis duration of up to 48 hours has been accepted without evidence to support the practice is an area that remains controversial. The duration of cardiothoracic prophylaxis in these guidelines is based on expert panel consensus because the available data do not delineate the optimal duration of prophylaxis. In these procedures, prophylaxis for the duration of the procedure and certainly for less than 24 hours is appropriate.
A 1992 meta-analysis of studies comparing first-generation cephalosporins and antistaphylococcal antimicrobials (e.g., penicillins) with second-generation cephalosporins in patients undergoing cardiothoracic surgery found a reduction in the rate of SSI with second-generation cephalosporins but no benefit from continuing surgical prophylaxis beyond 48 hours.125 Reports published in 1980,126 1993,127 1997,128 and 2000129 involving seven studies that compared single-dose prophylaxis or prophylaxis only during the operation with durations of one to four days failed to show any reduction in SSIs with the longer durations of prophylaxis. In a more-recent observational four-year cohort study of 2641 patients undergoing coronary artery bypass graft (CABG) surgery, the extended use of antimicrobial prophylaxis (>48 hours) instead of a shorter duration of prophylaxis (<48 hours) failed to reduce the risk of SSI (OR, 1.2; 95% CI, 0.8–1.6).130 Moreover, prolonged prophylaxis was associated with an increased risk of acquired antimicrobial resistance (cephalosporin-resistant Enterobacteriaceae and VRE) compared with short-term prophylaxis (OR, 1.6; 95% CI, 1.1–2.6).
There are no data to support the continuation of antimicrobial prophylaxis until all indwelling drains and intravascular catheters are removed.19,31,32,41,131–134
Topical Administration of Irrigations, Pastes, and Washes
I.V. and oral antimicrobial administration are the main focus of these guidelines, and these routes of administration are used for most surgical procedures addressed by these guidelines, with the exception of ophthalmic procedures, for which topical administration is the primary route of administration. Limited high-quality data are available regarding the use of antimicrobial irrigations, pastes, and washes that are administered topically. Studies published in the early 1980s demonstrated that prophylactic topical administration of antimicrobials in the surgical incision during various nonophthalmic procedures is superior to placebo but not superior to parenteral administration, and topical administration does not increase the efficacy of parenteral antimicrobials when used in combination for prophylaxis.135–138 Additional high-quality data on the safety and efficacy of topical antimicrobial administration as an adjunct to i.v. administration are needed to determine the role of topical antimicrobial prophylaxis.
One area of interest for topical administration of antimicrobials, mainly gentamicin and vancomycin, is application to the sternum during cardiac procedures in combination with i.v. agents to prevent mediastinitis. This strategy has been evaluated in cohort and randomized controlled studies.139–142 While the studies found a significantly lower rate of SSI with topical antimicrobials compared with standard prophylaxis,140 placebo,142 and a historical control,139 a smaller, randomized, placebo-controlled study found no difference between groups.141
More recently, implantable gentamicin collagen sponges failed to show any efficacy in reducing SSIs in a large prospective study of patients undergoing cardiac surgery and resulted in an increased infection rate in patients undergoing colectomy.143,144 The safety and efficacy of topical antimicrobials have not been clearly established; therefore, routine use of this route cannot be recommended in cardiac or other procedures.145
Preoperative Screening and Decolonization
S. aureus is the most common pathogen causing SSIs, accounting for 30% of SSIs in the United States. Colonization with S. aureus, primarily in the nares, occurs in roughly one in four persons and increases the risk of SSI by 2- to 14-fold.146–152 A national survey assessing nasal colonization with S. aureus in the general population conducted from 2001 through 2004 found that while the rate of colonization with S. aureus decreased from 32.4% in 2001–02 to 28.6% in 2003–04 (p < 0.01), the rate of colonization with MRSA increased from 0.8% to 1.5% (p < 0.05).75
Preoperative screening for S. aureus carriage and decolonization strategies have been explored as means to reduce the rate of SSIs. Anterior nasal swab cultures are most commonly used for preoperative surveillance, but screening additional sites (pharynx, groin, wounds, rectum) can increase detection rates.153 Such preoperative surveillance swabs that can be cultured on selective or nonselective media or sent for rapid polymerase chain reaction (PCR)-based screening can be used to identify colonized patients in the preoperative period. When properly used, all of these techniques can identify MSSA and MRSA. However, not all PCR-based systems will identify both MRSA and MSSA so verification with the laboratory is needed. While many studies have focused specifically on MRSA screening in high-risk hospitalized patients in an effort to prevent MRSA SSI and hospital-acquired infections, the risk of developing an SSI remains elevated for any S. aureus carrier. While some authors advocate screening for MRSA carriage in the general population, the data supporting universal screening in the surgical population are more controversial.154,155 Screening has been advocated to both identify candidates for S. aureus decolonization and inform the selection of optimal prophylactic antimicrobials, such as the addition of vancomycin for those colonized with MRSA.
FDA has approved intranasal mupirocin to eradicate MRSA nasal colonization in adult patients and health care workers.156 It is noted in the prescribing information that there are insufficient data to support use in prevention of autoinfection of high-risk patients from their own nasal colonization with S. aureus. However, additional data have demonstrated that the use of intranasal mupirocin in nasal carriers of S. aureus decreases the rate of S. aureus infections.157,158 One meta-analysis of seven studies focused on surgical patients only157; the other meta-analysis of nine studies included high-quality studies in dialysis patients.158
Recent studies have confirmed that S. aureus decolonization of the anterior nares decreases SSI rates in many surgical patients.159 The data are most compelling in cardiac and orthopedic surgery patients. There are fewer data in general surgery patients. A large, randomized controlled trial of general, cardiac, and neurosurgical patients (n = 3864) revealed that prophylactic intranasal application of mupirocin did not significantly reduce the overall rate of S. aureus SSIs (2.3% in the mupirocin group versus 2.4% in the control group) but did decrease the rate of S. aureus SSI among S. aureus carriers (3.7% in the mupirocin group versus 5.9% in the control group).160
Another randomized controlled trial found no significant difference in the rate of postoperative S. aureus SSIs among cardiac surgery patients receiving intranasal mupirocin and those receiving placebo, but the study was limited by the small numbers of patients (n = 257) and reported SSIs (n = 5).161 Among elective orthopedic patients undergoing implantation and other procedures, a randomized clinical trial demonstrated a nonsignificant reduction in the rate of postoperative S. aureus SSIs in patients receiving mupirocin (n = 315, 3.8%) compared with those receiving placebo (n = 299, 4.7%).150
A recent randomized, double-blind, placebo-controlled, multicenter study conducted in the Netherlands found that the use of mupirocin nasal ointment and chlorhexidine baths in identified S. aureus carriers reduced the risk of hospital-associated S. aureus infections.162 In the study, a real-time PCR assay was used to rapidly identify S. aureus nasal carriers; all of the S. aureus isolates were susceptible to methicillin. Deep SSIs occurred in 0.9% of the mupirocin–chlorhexidine-treated group (4 of 441 patients) versus 4.4% of the placebo group (16 of 367 patients) (relative risk, 0.21; 95% CI, 0.07–0.62). The reduction in superficial SSIs was less marked (1.6% versus 3.5%; relative risk, 0.45; 95% CI, 0.18–1.11). It is plausible that this approach would be beneficial in a setting of MRSA, but it has not been proven.
Most studies conclude that the use of preoperative intranasal mupirocin in colonized patients is safe and potentially beneficial as an adjuvant to i.v. antimicrobial prophylaxis to decrease the occurrence of SSIs. However, the optimal timing and duration of administration are not standardized. In most studies, mupirocin was used for five days before the operation. While S. aureus resistance to mupirocin has been detected,148,162 raising concerns about the potential for widespread problems with resistance from routine use of this agent, resistance has only rarely been seen in the preoperative setting. Low-level resistance is associated with an increased rate of failure of decolonization and has been seen in institutions that use standardized mupirocin decolonization protocols.163 Therefore, when decolonization therapy (e.g., mupirocin) is used as an adjunctive measure to prevent S. aureus SSI, surveillance of susceptibility of S. aureus isolated from SSIs to mupirocin is recommended.164 While universal use of mupirocin is discouraged, specific recommendations for the drug’s use can be found in the cardiac and orthopedic sections of these guidelines.
Future Research
Additional research is needed in several areas related to surgical antimicrobial prophylaxis. The risks and benefits of continuing antimicrobial prophylaxis after the conclusion of the operative procedure, including dosing and duration, need to be further evaluated. Insight is needed to make specific recommendations for intraoperative repeat dosing, weight-based dosing in obese patients, and timing of presurgical antimicrobials that must be administered over a prolonged period (e.g., vancomycin, fluoroquinolones). Additional clarification is needed regarding targeted antimicrobial concentrations and intraoperative monitoring of antimicrobial serum and tissue concentrations to optimize efficacy. The role of topical administration of antimicrobial agents as a substitute for or an adjunct to i.v. antimicrobial prophylaxis needs to be further evaluated. Additional data are needed to guide the selection of antimicrobial agents for prophylaxis, particularly combination regimens, for patients with allergies to β-lactam antimicrobials. Data are also needed to devise strategies to optimize antimicrobial prophylaxis in patients and facilities with a high risk or high prevalence of resistant organisms implicated in SSIs (e.g., MRSA). Optimal strategies for screening for S. aureus and decolonization for certain procedures need to be identified. Finally, outcomes studies are needed to assess the impact of using quality measures and pay-for-performance incentives designed to reduce surgical morbidity and mortality.
Cardiac Procedures
Background. Cardiac procedures include CABG procedures, valve repairs, and placement of temporary or permanent implantable cardiac devices, including ventricular assist devices (VADs). SSIs, including mediastinitis and sternal wound infection, are rare but serious complications after cardiac procedures. In patients undergoing CABG, the mean frequency of SSIs depending on NHSN SSI risk index category ranges from 0.35 to 8.49 per 100 operations when donor sites are included.165 The mean frequency of SSIs depending on NHSN SSI risk index category for patients undergoing CABG with only chest incisions ranges from 0.23 to 5.67 per 100 operations.165 Most of these infections are superficial in depth. Patient-related and procedure-related risk factors for SSIs after cardiac procedures have been identified from several single-center cohort and case–control studies.117,128,166–176 These include diabetes,166,169,171–175 hyperglycemia,177–182 peripheral vascular disease,171,172,174 chronic obstructive pulmonary disease,166,174,175 obesity (BMI of >30 kg/m2),166–168,171,173–176 heart failure,171,172 advanced age,117,128,166,172 involvement of internal mammary artery,168–172 reoperation,169–171 increased number of grafts,171 long duration of surgery,117,166,167,176 and S. aureus nasal colonization.146,160
Patients requiring extracorporeal membrane oxygenation (ECMO) as a bridge to cardiac or lung transplantation should be treated with a similar approach. If there is no history of colonization or previous infection, the general recommendations for SSI antimicrobial prophylaxis for the specific procedure should be followed. For ECMO patients with a history of colonization or previous infection, changing the preoperative antimicrobial prophylaxis to cover these pathogens must be considered, weighing whether the pathogen is relevant to SSIs in the planned procedure.
Organisms. Almost two thirds of organisms isolated in both adult and pediatric patients undergoing cardiac procedures are gram-positive, including S. aureus, coagulase-negative staphylococcus, and, rarely, Propionibacterium acnes. Gram-negative organisms are less commonly isolated in these patients and include Enterobacter species, Pseudomonas aeruginosa, Escherichia coli, Klebsiella pneumoniae, Proteus mirabilis, and Acinetobacter species.93,139,146,183–192
Efficacy. The SSI rate in cardiac procedures is low, but there are potential consequences if infection occurs. Multiple studies have found that antimicrobial prophylaxis in cardiac procedures lowers the occurrence of postoperative SSI up to fivefold.125
Choice of agent. Cephalosporins have been the most studied antimicrobials for the prevention of SSIs in cardiac procedures. Both first-generation (cefazolin) and second-generation (cefamandole and cefuroxime) cephalosporins have been shown to be effective in reducing SSI in cardiac surgery; however, the superiority of one class over another has not been proven.125,127,193–199
A meta-analysis comparing cephalosporins with glycopeptides (e.g., vancomycin) as antimicrobial prophylaxis regimens for cardiac procedures found a higher frequency of postoperative chest and deep-chest SSIs and a trend toward an increased risk of gram-positive SSI in the glycopeptide group but a lower frequency of SSIs caused by resistant gram-positive pathogens.72 The routine use of vancomycin for the prevention of SSIs is not recommended, based on limited evidence of efficacy and concerns of increased glycopeptide resistance of microorganisms.8,116 There is no clear evidence to support the use of vancomycin, alone or in combination with other antimicrobials, for routine antimicrobial prophylaxis in institutions that have a high prevalence of MRSA.8,11,41,72,73,116,200 Vancomycin should be considered in patients who are colonized with MRSA.41,116,201 The accepted alternative antimicrobial for β-lactam-allergic patients undergoing cardiac procedures is vancomycin or clindamycin for gram-positive coverage.41,116,201,202 The addition of an aminoglycoside, aztreonam, or a fluoroquinolone may be prudent when gram-negative pathogens are a concern.8,116
Mupirocin. The proportion of infections related to S. aureus among patients undergoing cardiac surgery and the increase in MRSA as a cause of SSIs at some institutions have led to investigations of methods for preoperative eradication, particularly with intranasal mupirocin.203 Readers are referred to the Common Principles section of these guidelines for discussion of the use of intranasal mupirocin. Of note, the data demonstrated a 45% reduction in S. aureus SSIs with the use of preoperative mupirocin among patients known to be colonized with S. aureus who undergo cardiac procedures.157,193 Institutions should monitor for mupirocin resistance periodically.
Topical administration. Additional information on topical administration of antimicrobials can be found in the Common Principles section of these guidelines. Use of topical antimicrobials, mainly gentamicin or vancomycin, applied to the sternum during cardiac procedures in combination with i.v. agents to prevent mediastinitis has been evaluated in both cohort139 and randomized controlled studies.140–142 While the studies found a significantly lower rate of SSIs with topical antimicrobials compared with standard prophylaxis,140 placebo,142 and a historical control,139 a smaller randomized, placebo-controlled study found no difference between groups.141 More recent studies of gentamicin collagen sponges failed to show any efficacy in a large prospective study of cardiac surgery.143 The safety and efficacy of topical antimicrobials have not been clearly established and therefore cannot be recommended for routine use in cardiac procedures.139–142
Cardiopulmonary bypass. Cardiopulmonary bypass (CPB) is a common surgical technique in cardiac procedures that alters the volume of distribution and bioavailability of medications administered during the procedure.116,204,205 Several small cohort or comparative studies128,204–213 have evaluated the serum and tissue concentrations of several routinely used antimicrobial prophylactic agents (i.e., cefazolin, cefuroxime, gentamicin, and vancomycin) in patients undergoing CPB during cardiac procedures. Until further clinical outcomes data and well-designed studies become available to inform alternative dosing strategies, routinely used doses of common antimicrobial agents should be used in patients undergoing CPB during cardiac procedures.
Duration. The optimal duration of antimicrobial prophylaxis for cardiac procedures continues to be evaluated. Data support a duration ranging from a single dose up to 24 hours postoperatively.41,99,131,191,214–217 No significant differences were found in several small studies in patients undergoing cardiac procedures between these dosing strategies in patients primarily receiving first- or second-generation cephalosporins. Although a recent meta-analysis suggested the possibility of increased efficacy with cardiac surgical prophylaxis extending beyond 24 hours, the authors noted that the findings were limited by the heterogeneity of antimicrobial regimens used and the risk of bias in the published studies.218 The comparisons of varying durations were performed with different antimicrobials with differing efficacy and do not support longer durations. Consequently, this meta-analysis does not provide evidence to support changing the currently accepted prophylaxis duration of less than 24 hours, particularly given the evidence from studies involving noncardiac operations. The currently accepted duration of prophylaxis for cardiac procedures is less than 24 hours, but prophylaxis should be continued for the duration of the procedure.41,59,126–129,131,201
Two small studies did not support the continuation of antimicrobial prophylaxis until intravascular catheters or intraaortic balloon pumps were removed, due to a lack of influence on infections or catheter colonization compared with short-course (24 hours) cefazolin or cefuroxime.219,220 The practice of continuing antimicrobial prophylaxis until all invasive lines, drains, and indwelling catheters are removed cannot be supported due to concerns regarding the development of drug-resistant organisms, superinfections, and drug toxicity.41,131
Pediatric Efficacy. The rate of SSI in pediatric cardiac procedures is sometimes higher than in adult patients.20,31,221 Significant risk factors in pediatric patients with a mediastinal SSI included the presence of other infections at the time of the procedure, young age (newborns and infants), small body size, the duration of the procedure (including CPB time), the need for an intraoperative blood transfusion, an open sternum postoperatively, the need for a reexploration procedure, the length of stay in the intensive care unit, an NNIS/NHSN risk score of 2, and the performance of emergency procedures.20,31,221
The organisms of concern in pediatric patients are the same as those in adult patients.20,21,31,221 However, MRSA is rarely a concern in this population as a risk factor for SSI.221 Pediatric patients considered at high risk for MRSA infection are those with preoperative MRSA colonization or a history of MRSA infection, neonates younger than one month of age, and neonates under three months of age who have been in the hospital since birth or have a complex cardiac disorder.21 Strategies such as intranasal mupirocin and changes in antimicrobial prophylactic agent to vancomycin led to decreased rates of MRSA carriage and the absence of MRSA infections in one time-series evaluation; however, the overall clinical impact of these efforts is still unclear.21,221
No well-controlled studies have evaluated the efficacy of antimicrobial prophylaxis in pediatric patients undergoing cardiac procedures. Therefore, the efficacy of antimicrobial prophylaxis is extrapolated from adult studies and should be considered the standard of care for pediatric cardiac surgery patients.19
No well-designed studies or consensus has established the appropriate doses for common antimicrobial prophylactic agents for use in pediatric cardiac patients. Antibiotic doses have been extrapolated from guidelines for the prevention of bacterial endocarditis.11 In recent evaluations, doses of cefazolin have ranged from 25 to 50 mg/kg,19–21,31 and vancomycin doses have ranged from 10 to 20 mg/kg.19–21,31,222–226 Gentamicin doses used in studies have included 2.520 and 5 mg/kg22; however, the study authors22 felt that the higher dose was excessive. The expert panel recognizes that the usual total daily dose for pediatric patients older than six months can be 6.5–7.5 mg/kg and that dosing schedules for younger patients may be complicated.
Recommendations. For patients undergoing cardiac procedures, the recommended regimen is a single preincision dose of cefazolin or cefuroxime with appropriate intraoperative redosing (Table 2). Currently, there is no evidence to support continuing prophylaxis until all drains and indwelling catheters are removed. Clindamycin or vancomycin is an acceptable alternative in patients with a documented β-lactam allergy. Vancomycin should be used for prophylaxis in patients known to be colonized with MRSA. If organizational SSI surveillance shows that gram-negative organisms cause infections for patients undergoing these operations, practitioners should combine clindamycin or vancomycin with another agent (cefazolin if the patient is not β-lactam allergic; aztreonam, aminoglycoside, or single-dose fluoroquinolone if the patient is β-lactam allergic). Mupirocin should be given intranasally to all patients with documented S. aureus colonization. (Strength of evidence for prophylaxis = A.)
Cardiac Device Insertion Procedures
Background. Antimicrobial prophylaxis is the standard of care for patients undergoing cardiac implantable device insertion (e.g., pacemaker implantation).227 Based on available data and perceived infection risk, antimicrobial prophylaxis is not routinely recommended for cardiac catheterization or transesophageal echocardiogram.228
NHSN has reported a mean SSI rate after pacemaker placement of 0.44 per 100 procedures.165 This rate may underestimate the risk of late SSI and complications.229 Risk factors for device-related infection after implantation of cardioverter–defibrillator systems or pacemakers identified in two large, prospective, multicenter cohort studies230,231 and a large case–control study232 included fever within 24 hours before implantation, temporary pacing before implantation, and early reintervention for hematoma or lead replacement230; corticosteroid use for more than one month during the preceding year and more than two leads in place compared with two leads232; and development of pocket hematoma.231 In all of the evaluations, antimicrobial prophylaxis was found to be protective against device-related infection.230–232 Limited data are available on the efficacy and optimal dose and duration of antimicrobial prophylaxis in patients undergoing implantation of a new pacemaker, pacing system, or other cardiac device.
A meta-analysis of 15 prospective, randomized, controlled, mainly open-label studies evaluated the effectiveness of systemic antimicrobial prophylaxis compared with controls (no antimicrobials) on infection rates after pacemaker implantation.227 Antibiotics included penicillins or cephalosporins with a duration ranging from a single preoperative dose to four days postoperatively. A consistent and significant protective effect of antimicrobial prophylaxis was found and encouraged the routine use of antimicrobial prophylaxis in patients undergoing permanent pacemaker implantation. A prospective, single-center cohort study found a low rate (1.7%) of SSI complications with a single 2-g dose of cefazolin in patients undergoing implantation of a new pacemaker, pulse-generator replacement, or upgrading of a preexisting pacing system.233 A notable limitation of the study was the exclusion of patients with temporary percutanous cardiac stimulators who are at high risk of infection.
A large, randomized, double-blind, placebo-controlled study found a significantly lower rate of SSI with a single 1-g dose of cefazolin (0.64%) compared with placebo (3.28%) (p = 0.016) given immediately before device implantation or generator replacement in a permanent pacemaker, implantable cardioverter defibrillator, or cardiac resynchronization device in a surgical operating room.231 The expert panel noted that the cefazolin dose was not adjusted for patient weight. Recently, AHA produced evidence-based guidelines that recommend the use of a single dose of a preoperative antimicrobial.229
VADs are increasingly used to bridge patients to transplantation or to support individuals who do not respond to medical therapy for congestive heart failure. Very limited data exist on infection rates, and there are no published studies that demonstrate the effectiveness of preoperative antimicrobial therapy. Using 2006–08 data from the Interagency Registry for Mechanically Assisted Circulatory Support, Holman and colleagues234 reported that most infections related to mechanical cardiac support devices were bacterial (87%), with the remainder associated with fungal (9%), viral (1%), protozoal (0.3%), or unknown (2%) causes. Driveline infections are primarily caused by staphylococcal species from the skin. Fungal organisms also play an important role in VAD infections, most notably Candida species, and carry a high risk of mortality. A recent survey of antimicrobial surgical prophylaxis with VADs illustrates the variability and lack of consensus with regimens, using anywhere from one to four drugs for a duration of 24–72 hours.235 Immediate postoperative infections are caused by gram-positive organisms. Complications from long-term infections should not be confused with immediate postprocedure SSIs.236 Based on the consensus of the expert panel, antimicrobial prophylaxis for replacement of a VAD due to ongoing or recent infection should incorporate coverage directed at the offending organism or organisms. While many centers use vancomycin plus ciprofloxacin plus fluconazole, this practice is not based on the published evidence.
Recommendation. A single dose of cefazolin or cefuroxime is recommended for device implantation or generator replacement in a permanent pacemaker, implantable cardioverter defibrillator, or cardiac resynchronization device. (Strength of evidence for prophylaxis = A.) There is limited evidence to make specific recommendations for VADs, and each practice should tailor protocols based on pathogen prevalence and local susceptibility profiles. Clindamycin or vancomycin is an acceptable alternative in patients with a documented β-lactam allergy. Vancomycin should be considered for prophylaxis in patients known to be colonized with MRSA.
Thoracic Procedures
Background. Noncardiac thoracic procedures include lobectomy, pneumonectomy, thoracoscopy, lung resection, and thoracotomy. In addition to SSIs, postoperative nosocomial pneumonia and empyema are of concern after thoracic procedures.237
NHSN has reported that the rate of infection associated with thoracic surgery ranges from 0.76% to 2.04%.165 Studies have found that the reported rate of SSIs after thoracic procedures in patients receiving antimicrobial prophylaxis ranged from 0.42% to 4%.238–241 One study found an SSI rate of 14% when prophylaxis was not used.239 The reported rates of pneumonia and empyema with antimicrobial prophylaxis are 3–24% and 0–7%, respectively.237,239–244
Video-assisted thoracoscopic surgery (VATS) is commonly used for thoracic procedures. In some settings, VATS constitutes one third or more of all thoracic surgical procedures.245 Since VATS uses small incisions, the rate of SSIs is lower compared with the rate associated with open thoracic surgical procedures.246 A prospective cohort study (n = 346) confirmed a low rate of SSIs (1.7%) after minimally invasive VATS procedures.240 An additional prospective study of 988 lung resection patients confirmed that the SSI rate was significantly lower (5.5%) in VATS patients than in open thoracotomy patients (14.3%).247 Furthermore, SSI correlated with the duration of surgery, serum albumin, concurrent comorbidity, age, and forced expiratory volume in one second. Antimicrobial prophylaxis recommendations in this section refer to both open thoracotomy and VATS procedures. Based on available data and perceived infection risk, antimicrobial prophylaxis is not routinely recommended for chest tube insertion.
Results of a prospective cohort and case–control study revealed the following independent risk factors for pneumonia after thoracic procedures: extent of lung resection, intraoperative bronchial colonization, chronic obstructive pulmonary disease, BMI of >25 kg/m2, induction therapy (chemotherapy, radiotherapy, or chemoradiotherapy), advanced age (≥75 years old), and stage III or IV cancer.243,244
Organisms. The organisms reported from SSIs in patients undergoing thoracic procedures were S. aureus and S. epidermidis.237 Organisms isolated in patients with postoperative pneumonia included gram-positive (Streptococcus and Staphylococcus species), gram-negative (Haemophilus influenzae, Enterobacter cloacae, K. pneumoniae, Acinetobacter species, P. aeruginosa, and Moraxella catarrhalis), and fungal (Candida species) pathogens.237,239–243
Efficacy. Antimicrobial prophylaxis is the standard of care for patients undergoing noncardiac thoracic surgery, including pulmonary resection.11,201,237 One randomized, double-blind, placebo-controlled, single-center study of patients in Spain undergoing pulmonary resection, persistent pneumothorax without thoracotomy tube before surgery, and nonpulmonary thoracic surgical procedures, excluding those involving the esophagus and exploratory thoracotomies, compared a single dose of cefazolin 1 g i.v. and placebo given 30 minutes before the procedure.239 The study was stopped early due to the significant difference in SSI rates between groups (1.5% with cefazolin versus 14% with placebo, p < 0.01). No differences in the rates of pneumonia and empyema were seen between groups, but these were not endpoints of the study.
Choice of agent. There is no clear optimal choice for antimicrobial prophylaxis in thoracic procedures. The need to consider pneumonia and empyema as well as SSIs after thoracic procedures has been raised in the literature.237,241–244 There are a limited number of small, single-center, randomized controlled or cohort studies that evaluated several antimicrobial agents. One small, randomized controlled study and one cohort study found that ampicillin–sulbactam was significantly better than cephalosporins (cefazolin and cefamandole) for preventing pneumonia.242,243 No statistically significant difference was found between cefuroxime and cefepime in the rate of postoperative SSI, pneumonia, or empyema in a small, randomized controlled study in patients undergoing elective thoracotomy.241 Lower rates of infections and susceptibility of all organisms were noted with cefuroxime compared with cefepime. Therefore, the study authors concluded that cefuroxime was marginally more effective and was more cost-effective than cefepime.
Duration. No clear consensus on the duration of antimicrobial prophylaxis has been established. Studies have evaluated different dosing strategies for cephalosporins or penicillins, with most studies using single doses given preoperatively within 60 minutes before surgical incision.237,239,240,242,244 Studies found differing results when comparing agents given for 24 hours (cefepime, ampicillin–sulbactam) and 48 hours (cefuroxime, cefamandole); however, these findings may be attributable to the different antimicrobials tested.241,243 Additional discussion on dosing is provided in the Common Principles section of these guidelines.
Recommendations. In patients undergoing thoracic procedures, a single dose of cefazolin or ampicillin–sulbactam is recommended (Appendix B). Clindamycin or vancomycin is an acceptable alternative in patients with a documented β-lactam allergy. Vancomycin should be used for prophylaxis in patients known to be colonized with MRSA. If organizational SSI surveillance shows that gram-negative organisms are associated with infections during these operations or if there is risk of gram-negative contamination of the surgical site, practitioners should combine clindamycin or vancomycin with another agent (cefazolin if the patient is not β-lactam allergic; aztreonam, aminoglycoside, or single-dose fluoroquinolone if the patient is β-lactam allergic). (Strength of evidence for prophylaxis for VATS = C; strength of evidence for prophylaxis for other thoracic procedures = A.)
Gastroduodenal Procedures
Background. The gastroduodenal procedures considered in these guidelines include resection with or without vagotomy for gastric or duodenal ulcers, resection for gastric carcinoma, revision required to repair strictures of the gastric outlet, percutaneous endoscopic gastrostomy (PEG) insertion, perforated ulcer procedures (i.e., Graham patch repair), pancreaticoduodenectomy (Whipple procedure), and bariatric surgical procedures (gastric bypass, gastric banding, gastroplasty, other restrictive procedures, biliopancreatic diversion). Studies specifically addressing antimicrobial prophylaxis for gastroesophageal reflux disease procedures (Nissen fundoplication) or highly selective vagotomy for ulcers (usually done laparoscopically) could not be identified. Antireflux procedures and highly selective vagotomy are clean procedures in contrast to essentially all other gastroduodenal procedures that are clean-contaminated. Other procedures that are generally performed using laparoscopic or endoscopic techniques (e.g., endoscopic retrograde cholangiopancreatography) are not specifically discussed in this document. Natural orifice transluminal endoscopic surgery (NOTES) is a developing operative technique using natural orifices (e.g., vagina, anus, mouth, stomach) for entry into the abdomen that leaves no visible scar.248 No studies on antimicrobial prophylaxis using NOTES have been published. SSI rates reported in patients not receiving antimicrobial prophylaxis were 6% after vagotomy and drainage, 13% after gastric ulcer procedures, 6.8–17% after procedures for gastric cancer,249–253 8% for pancreaticoduodenectomy,254 and 23.9–26% after PEG insertion.255,256
The stomach is an effective barrier to bacterial colonization; this is at least partially related to its acidity. The stomach and the duodenum typically contain small numbers of organisms (<104 colony-forming units [CFU]/mL), the most common of which are streptococci, lactobacilli, diphtheroids, and fungi.257,258 Treatment with agents that increase gastric pH increases the concentration of gastric organisms.259–261 Alterations in gastric and duodenal bacterial flora as a result of increases in gastric pH have the potential to increase the postoperative infection rate.262,263
The risk of postoperative infection in gastroduodenal procedures depends on a number of factors, including the gastroduodenal procedure performed. Patients who are at highest risk include those with achlorhydria, including those receiving pharmacotherapy with histamine H2-receptor antagonists or proton-pump inhibitors,264 gastroduodenal perforation, decreased gastric motility, gastric outlet obstruction, morbid obesity, gastric bleeding, or cancer.265 Similar to other types of surgical procedures, risk factors for SSIs related to gastroduodenal procedures include long procedure duration,252,266,267 performance of emergency procedures,250,261 greater than normal blood loss,251,252 American Society of Anesthesiologists (ASA) classification of ≥3, and late administration of antimicrobials.268
Organisms. The most common organisms cultured from SSIs after gastroduodenal procedures are coliforms (E. coli, Proteus species, Klebsiella species), staphylococci, streptococci, enterococci, and occasionally Bacteroides species.101,269–276
Efficacy. Randomized controlled trials have shown that prophylactic antimicrobials are effective in decreasing postoperative infection rates in high-risk patients after gastroduodenal procedures. The majority of available studies were conducted in single centers outside of the United States. Relative to other types of gastrointestinal tract procedures, the number of clinical trials evaluating antimicrobial prophylaxis for gastroduodenal procedures is limited. In placebo-controlled trials, infection rates ranged from 0% to 22% for patients receiving cephalosporins or penicillins and from 1.7% to 66% for patients receiving placebo.270,271,273–275,277–284 The difference was significant in most studies.
Data support antimicrobial prophylaxis for patients undergoing PEG insertion.264,285–287 A Cochrane review of systemic antimicrobial prophylaxis for PEG procedures that included 11 randomized controlled trials and 1196 patients found a statistically significant reduction in peristomal infections with antimicrobial prophylaxis (OR, 0.35; 95% CI, 0.23–0.48).288 Two meta-analyses found statistically significant decreases in SSIs with antimicrobial prophylaxis compared with placebo or controls, from 23.9–26% to 6.4–8%, respectively.255,256 Most well-designed, randomized controlled studies found a significant decrease in postoperative SSIs or peristomal infections with single i.v. doses of a cephalosporin or penicillin, ranging from 11% to 17%, compared with from 18% to 66% with placebo or no antimicrobials.279–282,288 Conflicting results have been seen in studies evaluating the use of preoperative patient MRSA screening, decontamination washes and shampoos, five-day preoperative treatment with intranasal mupirocin, and single-dose teicoplanin preoperative prophylaxis to decrease postoperative MRSA infections during PEG insertion.289,290
While there have been no well-designed clinical trials of antimicrobial prophylaxis for patients undergoing bariatric surgical procedures, treatment guidelines support its use based on morbid obesity and additional comorbidities as risk factors for postoperative infections.264,291 There is no consensus on the appropriate antimicrobial regimen; however, higher doses of antimicrobials may be needed for adequate serum and tissue concentrations in morbidly obese patients.13,268,291
A notable risk factor for SSIs after esophageal and gastroduodenal procedures is decreased gastric acidity and motility resulting from malignancy or acid-suppression therapy.264,276 Therefore, antimicrobial prophylaxis is indicated for patients undergoing gastric cancer procedures (including gastrectomy) and gastroduodenal procedures related to gastric and duodenal ulcer disease or bariatric surgery or pancreaticoduodenectomy. Evaluations of practice for pancreaticoduodenectomy show that antimicrobials are typically given due to concerns of bile contamination. Prophylaxis for gastroduodenal procedures that do not enter the gastrointestinal tract, such as antireflux procedures, should be limited to high-risk patients due to lack of data supporting general use in all patients. Furthermore, laparoscopic antireflux procedures are associated with very low SSI rates (0.3%) compared with open antireflux procedures (1.4%), just as laparoscopic gastric bypass procedures are associated with lower rates than in open procedures (0.4% versus 1.2%).292
Choice of agent. The most frequently used agents for gastroduodenal procedures were first-generation271,273,277,278,284,293–297 and second-generation269,270,274,275,280,293,294,298 cephalosporins. No differences in efficacy between first- and second-generation cephalosporins were found. Amoxicillin–clavulanate279,282,283,299 and ciprofloxacin269,300 were also evaluated with similar results. Relatively few studies have compared the efficacy of different agents in reducing postoperative infection rates.
One meta-analysis recommended using a single dose of an i.v. broad-spectrum antimicrobial for SSI prophylaxis in these patients,256 while another found no differences between penicillin- or cephalosporin-based regimens and three-dose or single-dose regimens.255 In a comparative study, oral or i.v. ciprofloxacin and i.v. cefuroxime were similarly effective in upper gastrointestinal procedures, including gastrectomy, vagotomy, and fundoplication.300 No differences in efficacy were seen between ceftriaxone and combination ceftriaxone and metronidazole for PEG insertion in pediatric patients.301 An open-label study found a significant decrease in local peristomal and systemic infection (i.e., pneumonia) after PEG insertion after a single 1-g i.v. dose of ceftriaxone was given 30 minutes before surgery when compared with placebo (13.3% and 36.3%, respectively; p < 0.05).281 No differences were noted between cefotaxime and piperacillin–tazobactam for PEG SSIs.288 Ampicillin–sulbactam and cefazolin had equal efficacy in gastrectomy.253 One study found that piperacillin–tazobactam in combination with ciprofloxacin or gentamicin was the most active regimen against bacteria recovered from bile in pancreatoduodenectomy patients.302
Duration. The majority of studies evaluated a single dose of cephalosporin or penicillin.256,279–284,288,290,297 The available data indicate that single-dose and multiple-dose regimens are similarly effective. Three studies compared single- and multiple-dose regimens of cefamandole,294 amoxicillin–cluvulanate,299 and ampicillin–sulbactam and cefazolin.253 There were no significant differences in SSI rates. Multiple-dose regimens of first-generation (cefazolin) or second-generation (cefotiam) cephalosporins of four days, operative day only, and three days in duration did not differ in overall SSI rates.295
Recommendations. Antimicrobial prophylaxis in gastroduodenal procedures should be considered for patients at highest risk for postoperative infections, including risk factors such as increased gastric pH (e.g., patients receiving acid-suppression therapy), gastroduodenal perforation, decreased gastric motility, gastric outlet obstruction, gastric bleeding, morbid obesity, ASA classification of ≥3, and cancer.
A single dose of cefazolin is recommended in procedures during which the lumen of the intestinal tract is entered (Table 2). (Strength of evidence for prophylaxis = A.) A single dose of cefazolin is recommended in clean procedures, such as highly selective vagotomy, and antireflux procedures only in patients at high risk of postoperative infection due to the presence of the above risk factors. (Strength of evidence for prophylaxis = C.) Alternative regimens for patients with β-lactam allergy include clindamycin or vancomycin plus gentamicin, aztreonam, or a fluoroquinolone. Higher doses of antimicrobials are uniformly recommended in morbidly obese patients undergoing bariatric procedures. Higher doses of antimicrobials should be considered in significantly overweight patients undergoing gastroduodenal and endoscopic procedures.
Biliary Tract Procedures
Background. Biliary tract procedures include cholecystectomy, exploration of the common bile duct, and choledochoenterostomy. These guidelines pertain only to patients undergoing biliary tract procedures with no evidence of acute biliary tract infection and to patients with community-acquired acute cholecystitis of mild-to-moderate severity. As noted in the Common Principles section, patients receiving therapeutic antimicrobials for an infection before surgery should be given additional antimicrobial prophylaxis before surgery.
These guidelines do not address patients requiring biliary tract procedures for more-severe infections, including community-acquired acute cholecystitis with severe physiological disturbance, advanced age, or immunocompromised state; acute cholangitis; and health-care-associated or nosocomial biliary infections. These biliary tract infections are treated as complicated intraabdominal infections.303 All patients with a suspected biliary tract infection who undergo biliary tract surgery should receive preoperative i.v. antimicrobials.
The majority of published literature regarding SSIs in biliary tract procedures focuses on cholecystectomy. The overall reported rate of postoperative infection in open biliary tract procedures with antimicrobial prophylaxis is 1–19%.292,304–311 Infection rates after laparoscopic cholecystectomy range from 0% to approximately 4% in patients without antimicrobial prophylaxis308,312–320 and from 0% to 7% with prophylaxis.292,304–323 Several studies found that laparoscopic cholecystectomy SSI rates were significantly lower than those associated with open cholecystectomy.292,306–311
Risk factors associated with postoperative SSIs after biliary procedures include performance of emergency procedures,305 diabetes,305,306,311,315,317 longer procedure duration (over 120 minutes),305,317,324 intraoperative gallbladder rupture,305 age of >70 years,6,311,315,317,325 open cholecystectomy,7,311 conversion of laparoscopic to open cholecystectomy,7 higher ASA classification (≥3),306,310,317 episode of biliary colic within 30 days before the procedure,315,316 reintervention in less than a month for noninfectious complications,310 acute cholecystitis,6,7,306 bile spillage,7 jaundice,6,7,306 pregnancy,7 nonfunctioning gallbladder,6 and immunosuppression.7
The biliary tract is usually sterile. Patients with bacteria in the bile at the time of surgery may be at higher risk of postoperative infection305,326,327; however, some studies have found no association between the presence of bacteria in the bile and infection.305,315,316,319,321 Obesity (a BMI of >30 kg/m2) was found to be a risk factor in some studies306 but not in others.315,319 Laparoscopic cholecystectomy was associated with a significantly decreased risk for SSI.292,310,324,325
Organisms. The organisms most commonly associated with infection after biliary tract procedures include E. coli, Klebsiella species, and enterococci; less frequently, other gram-negative organisms, streptococci, and staphylococci are isolated.305,306,312,315,316,318,319,321,326,328–338 Anaerobes are occasionally reported, most commonly Clostridium species.
Recent studies have documented increasing antimicrobial resistance in the causative pathogens in biliary tract infections and other intra-abdominal infections, with up to 40% of E. coli isolates resistant to ampicillin–sulbactam and fluoroquinolones.339–341 Due to this increasing resistance of E. coli to fluoroquinolones and ampicillin–sulbactam, local population susceptibility profiles should be reviewed to determine the optimal antimicrobials for SSI prevention in biliary tract procedures.
Efficacy. Numerous studies have evaluated the use of prophylactic antimicrobials during biliary tract procedures, with a focus on laparoscopic cholecystectomy. Laparoscopic cholecystectomy has replaced open cholecystectomy as the standard of practice because of the reduction in recovery time and shorter hospital stay. The majority of studies of antimicrobial prophylaxis for laparoscopic cholecystectomy were underpowered and varied in control groups used (placebo, active, or no treatment), follow-up (from 30 to 60 days, while some studies did not clearly define length of time), and how SSIs were detected and reported.308,312–316,318,319,321,322 Some studies included patients who were converted from laparoscopic to open cholecystectomy and others did not.
A large, multicenter, quality-assurance study in Germany assessed the effectiveness of antimicrobial prophylaxis in laparoscopic and open cholecystectomies.308 This study included 4477 patients whose antimicrobial choice and dosage regimens were at the discretion of the medical center and surgeon. Antimicrobials used included first-, second-, and third-generation cephalosporins or penicillins alone or in combination with metronidazole, gentamicin, or both metronidazole and gentamicin. The most common cephalosporin used was ceftriaxone, allowing its data to be separated from data for other antimicrobials. Antimicrobial prophylaxis was administered to 2217 patients (ceftriaxone [n = 787 laparoscopic and n = 188 open] and other antimicrobials [n = 229 laparoscopic and n = 229 open]); none was given to 1328 laparoscopic and 932 open cholecystectomy patients. Significantly lower overall infectious complications occurred in patients receiving antimicrobial prophylaxis (0.8% ceftriaxone and 1.2% other antimicrobials), compared with 5% of those who received no prophylaxis (p < 0.05). The overall rates of infectious complications were 0.6%, 0.8%, and 3.3% in patients undergoing laparoscopic cholecystectomy receiving ceftriaxone, other antimicrobials, and no prophylaxis, respectively, and 1.6%, 3.9%, and 7.4%, respectively, for patients undergoing open cholecystectomy. Significantly lower rates of SSIs and postoperative pneumonia were noted in patients receiving antimicrobials compared with those who did not receive prophylaxis (p < 0.05). SSI rates were significantly decreased in laparoscopic cholecystectomy patients who received ceftriaxone (0.1%) or other antimicrobials (0.2%) compared with those who received no antimicrobial prophylaxis (1.6%). SSI rates were significantly decreased in open cholecystectomy patients who received ceftriaxone (1.0%) or other antimicrobials (2.6%) compared with those who received no antimicrobial prophylaxis (4.4%). The study authors concluded that antimicrobial prophylaxis should be administered to all patients undergoing cholecystectomy, regardless of approach. The study had several limitations, including lack of randomization, lack of adequate controls, and lack of clear definition of patient selection for the antimicrobial regimens. The statistical analysis was not clearly defined. The study appears to have compared only the use and lack of use of antimicrobials (with ceftriaxone and other antimicrobials combined for analysis) and did not specifically compare the laparoscopic and open approaches.
The findings of this study contrast with those of several other published studies. A meta-analysis of 15 randomized controlled studies evaluated the need for antimicrobial prophylaxis in elective laparoscopic cholecystectomy for patients at low risk of infection.313 Low risk was defined as not having any of the following: acute cholecystitis, a history of acute cholecystitis, common bile duct calculi, jaundice, immune suppression, and prosthetic implants. A total of 2961 patients were enrolled in the studies, including 1494 who received antimicrobial prophylaxis, primarily with cephalosporins, vancomycin, fluoroquinolones, metronidazole, and amoxicillin–clavulanate, and 1467 controls receiving placebo or no treatment. No significant difference was found in the rates of infectious complications (2.07% in patients receiving antimicrobial prophylaxis versus 2.45% in controls) or SSIs (1.47% in patients receiving antimicrobial prophylaxis versus 1.77% in controls). The authors of the meta-analysis concluded that antimicrobial prophylaxis was not necessary for low-risk patients undergoing elective laparoscopic cholecystectomy. An additional meta-analysis of 9 randomized controlled trials (n = 1437) also concluded that prophylactic antimicrobials do not prevent infections in low-risk patients undergoing laparoscopic cholecystectomy.342
A small, prospective, nonrandomized study compared the use of cefotaxime 1 g i.v. during surgery with an additional two i.v. doses given eight hours apart after surgery (n = 80) with no antimicrobial prophylaxis (n = 86) in patients undergoing elective laparoscopic cholecystectomy with accidental or incidental gallbladder rupture and spillage of bile.317 Patients who had spillage of gallstone calculi or whose operations were converted to open operations were excluded from the study. The rate of SSIs did not significantly differ between treatment groups (2.5% with antimicrobials versus 3.4% without antimicrobial prophylaxis). Based on results of multivariate analysis, routine antimicrobial prophylaxis was not recommended for these patients unless they were diabetic, were older than 60 years, or had an ASA classification of ≥3 or the duration of the procedure exceeded 70 minutes.
Current data do not support antimicrobial prophylaxis for low-risk patients undergoing elective laparoscopic cholecystectomies or those with incidental or accidental gallbladder rupture. Antimicrobial prophylaxis should be considered for patients at high risk of infection, including those undergoing open cholecystectomy, as described above, or who are considered to be at high risk for conversion to an open procedure.
Choice of agent. The data do not indicate a significant difference among first-, second-, and third-generation cephalosporins. First-generation,307,308,312,315,319,323,330,336,338,343,344 second-generation,308,314,315,318,323,327–329,331,332,335,344–352 and third-generation308,309,315–317,321,322,332,333,338,349,353,354 cephalosporins have been studied more extensively than other antimicrobials. Limited data are available for ampicillin with gentamicin,355 piperacillin,356 amoxicillin–clavulanate,305,338,351,354 ciprofloxacin,320,333,352,357 and cephalosporins or penicillins alone or in combination with metronidazole, gentamicin, or both metronidazole and gentamicin.308
Several studies have compared first-generation cephalosporins with second- or third-generation agents.315,336,338,344–347,353,358 With one exception,347 there was no significant difference in efficacy among agents. Other studies found no significant differences in efficacy between ampicillin and cefamandole,335 ciprofloxacin and ceftriaxone,333 amoxicillin–clavulanate and cefotaxime,354 amoxicillin–clavulanate and cefamandole,351 ceftriaxone and ceftazidime,321 and oral and i.v. ciprofloxacin and i.v. cefuroxime.352,357 One study found that i.v. ampicillin–sulbactam was associated with significantly lower rates of infection compared with cefuroxime306 and that patients treated with oral ceftibuten had significantly lower infection rates than those who received amoxicillin–clavulanate.338
Duration. The effect of duration of prophylaxis on outcome has been evaluated. A single dose of a cephalosporin was compared with multiple doses in several studies; no significant differences in efficacy were found.327,329,330,348,349,353,359 The largest study compared one dose of cefuroxime with three doses in 1004 patients with risk factors for infection who were undergoing biliary tract surgery.327 There was no significant difference in the rates of minor or major SSIs between the single- and multiple-dose groups. In the majority of studies, one dose of an antimicrobial was administered at induction of anesthesia,306,312,338,352,354 within 30 minutes before incision,338 or 1315,316,320,321 or 2338 hours before incision. Additional doses were given as follows: one dose 12 hours after administration of the initial dose,352 two doses 12 and 24 hours after administration of the initial dose,338 two doses every 6338 or 8317,319 hours after surgery, and one dose 24 hours after surgery315 and five days after surgery.352 In one study, a second dose of amoxicillin–clavulanate or cefotaxime was administered for procedures lasting longer than 4 hours.354
Recommendations. A single dose of cefazolin should be administered in patients undergoing open biliary tract procedures (Table 2). (Strength of evidence for prophylaxis = A.) Alternatives include ampicillin–sulbactam and other cephalosporins (cefotetan, cefoxitin, and ceftriaxone). Alternative regimens for patients with β-lactam allergy include clindamycin or vancomycin plus gentamicin, aztreonam, or a fluoroquinolone; or metronidazole plus gentamicin or a fluoroquinolone.
Antimicrobial prophylaxis is not necessary in low-risk patients undergoing elective laparoscopic cholecystectomies. (Strength of evidence against prophylaxis for low-risk patients = A.) Antimicrobial prophylaxis is recommended in patients undergoing laparoscopic cholecystectomy who have an increased risk of infectious complications. Risk factors include performance of emergency procedures, diabetes, anticipated procedure duration exceeding 120 minutes, risk of intraoperative gallbladder rupture, age of >70 years, open cholecystectomy, risk of conversion of laparoscopic to open cholecystectomy, ASA classification of ≥3, episode of biliary colic within 30 days before the procedure, reintervention in less than a month for noninfectious complications of prior biliary operation, acute cholecystitis, anticipated bile spillage, jaundice, pregnancy, nonfunctioning gallbladder, and immunosuppression. Because some of these risk factors cannot be determined before the surgical intervention, it may be reasonable to give a single dose of antimicrobial prophylaxis to all patients undergoing laparoscopic cholecystectomy. (Strength of evidence for prophylaxis for high-risk patients = A.)
Appendectomy Procedures
Background. Cases of appendicitis can be described as complicated or uncomplicated on the basis of the pathology. Patients with uncomplicated appendicitis have an acutely inflamed appendix. Complicated appendicitis includes perforated or gangrenous appendicitis, including peritonitis or abscess formation. Because complicated appendicitis is treated as a complicated intra-abdominal infection,303 it has not been addressed separately in these guidelines. All patients with a suspected clinical diagnosis of appendicitis, even those with an uncomplicated case, should receive appropriate preoperative i.v. antimicrobials for SSI prevention, which, due to the common microbiology encountered, requires similar antimicrobial choices to those used to treat complicated appendicitis.
Approximately 80% of patients with appendicitis have uncomplicated disease.59 SSI has been reported in 9–30% of patients with uncomplicated appendicitis who do not receive prophylactic antimicrobials, though some reports suggest lower complication rates in children with uncomplicated appendicitis.165,360–365 Mean SSI rates for appendectomy reported in the most recent NHSN report (2006–08) were 1.15% (60 of 5211) for NHSN risk index categories 0 and 1 versus 3.47% (23 of 663) for NHSN risk index categories 2 and 3.165 Laparoscopic appendectomy has been reported to produce lower rates of incisional (superficial and deep) SSIs than open appendectomy in adults and children in multiple meta-analyses and several randomized clinical trials.292,310,366–371 However, the rate of organ/space SSIs (i.e., intra-abdominal abscesses) was significantly increased with laparoscopic appendectomy.
Organisms. The most common microorganisms isolated from SSIs after appendectomy are anaerobic and aerobic gram-negative enteric organisms. Bacteroides fragilis is the most commonly cultured anaerobe, and E. coli is the most frequent aerobe, indicating that the bowel flora constitute a major source for pathogens.59,372,373 Aerobic and anaerobic streptococci, Staphylococcus species, and Enterococcus species also have been reported. P. aeruginosa has been reported infrequently.
Efficacy. Antibiotic prophylaxis is generally recognized as effective in the prevention of postoperative SSIs in patients undergoing appendectomy when compared with placebo.374
Choice of agent. Randomized controlled trials have failed to identify an agent that is clearly superior to other agents in the prophylaxis of postappendectomy infectious complications. An appropriate choice for SSI prophylaxis in uncomplicated appendicitis would be any single agent or combination of agents that provides adequate gram-negative and anaerobic coverage. The second-generation cephalosporins with anaerobic activity and a first-generation cephalosporin plus metronidazole are the recommended agents on the basis of cost and tolerability. Given the relatively equivalent efficacy between agents, a cost-minimization approach is reasonable; the choice of agents should be based on local drug acquisition costs and antimicrobial sensitivity patterns.
A wide range of antimicrobials have been evaluated for prophylaxis in uncomplicated appendicitis. The most commonly used agents were cephalosporins. In general, a second-generation cephalosporin with anaerobic activity (cefoxitin or cefotetan) or third-generation cephalosporins with partial anaerobic activity (cefotaxime) were effective, with postoperative SSI rates of <5% in most studies.364,375–381
Piperacillin 2 g was comparable to cefoxitin 2 g in a well-controlled study.381 Metronidazole used alone was less effective than cefotaxime, with infection rates above 10%.376 However, when metronidazole was combined with cefazolin, ampicillin,382 or gentamicin,378,383 the post-operative SSI rates were 3–6%.
A double-blind, randomized, controlled trial was conducted at two hospitals to evaluate the effect of metronidazole, which is effective against most anaerobes, and cefazolin, which is effective against many aerobic organisms, singly and in combination, on the rate of sepsis after appendectomy.384 Patients were randomized into one of four groups: metronidazole and placebo, cefazolin and placebo, metronidazole and cefazolin, or double placebo. Patients with generalized peritonitis were excluded for ethical reasons. Treatment was started before the procedure and continued every 8 hours for 24 hours. All patients in the trial were followed for about two weeks after discharge from the hospital, and their surgical sites were inspected. A total of 271 patients were assessed. Sepsis rates at the two hospitals were similar. Patients who received both cefazolin and metronidazole had a significantly lower infection rate compared with the other groups.384 Consistent with the antibacterial spectrum of the agents, a prospective study of antimicrobial prophylaxis for colorectal procedures found that the combination of metronidazole with aztreonam did not show adequate coverage of gram-positive organisms.385 The Common Principles section of these guidelines provides additional considerations for weight-based dosing.
Duration. In most of the studies of second- or third-generation cephalosporins or metronidazole combinations, a single dose376–378,380,383 or two or three doses364,379,382 were given. Although direct comparisons were not made, there was no discernible difference in postoperative SSI rates between single-dose and multidose administration in most studies. A randomized trial specifically comparing different durations of regimens found no statistical difference between a single preoperative dose, three doses (preoperative dose plus two additional doses), or a five-day regimen.386 A large cohort study found that single doses of metronidazole and gentamicin in patients undergoing open appendectomy were effective and sufficient in decreasing the SSI rate.387
Pediatric Efficacy. In pediatric patients, as with adults, preoperative determination of complicated versus uncomplicated appendicitis is difficult. A comprehensive review is not provided here, but this topic has been addressed by SIS.388
Two pediatric studies demonstrated no difference in SSI rates between placebo and several antimicrobials. The first study compared metronidazole, penicillin plus tobramycin, and piperacillin.389 The second study compared single-dose metronidazole and single-dose metronidazole plus cefuroxime.390 A meta-analysis including both adult and pediatric studies found that for pediatric patients, antimicrobial prophylaxis trended toward being beneficial, but the results were not statistically significant.374 A retrospective chart review questioned the routine need for antimicrobial prophylaxis in children with simple appendicitis, due to relatively low infection rates in children not receiving prophylaxis.365 However, these and other study authors have suggested antimicrobial prophylaxis may be considered due to the morbidity associated with infectious complications (e.g., prolonged hospitalization, readmission, reoperation) and due to the inability to preoperatively identify appendicitis.
As a single agent, metronidazole was no more effective than placebo in two double-blind studies that included children 10 years of age or older360 and 15 years of age or older.363 In a randomized study that included pediatric patients, ceftizoxime and cefamandole were associated with significantly lower infection rates and duration of hospitalization than placebo.391 Both cefoxitin and a combination of gentamicin and metronidazole were associated with a lower rate of postoperative infection in a randomized study that included pediatric patients younger than 16 years.378 Second-generation cephalosporins with anaerobic activity (cefoxitin or cefotetan) and third-generation cephalosporins with anaerobic activity (cefotaxime) were effective, with post-operative infection rates of <5% in two studies that included pediatric patients younger than 12 years.364,378,379 A single dose of gentamicin with clindamycin was found to be safe and effective in children with simple appendicitis.392
Recommendations. For uncomplicated appendicitis, the recommended regimen is a single dose of a cephalosporin with anaerobic activity (cefoxitin or cefotetan) or a single dose of a first-generation cephalosporin (cefazolin) plus metronidazole (Table 2). For β-lactam-allergic patients, alternative regimens include (1) clindamycin plus gentamicin, aztreonam, or a fluoroquinolone and (2) metronidazole plus gentamicin or a fluoroquinolone (ciprofloxacin or levofloxacin). (Strength of evidence for prophylaxis = A.)
Background. Small intestine procedures, or small bowel surgery as defined by NHSN, include incision or resection of the small intestine, including enterectomy with or without intestinal anastomosis or enterostomy, intestinal bypass, and strictureoplasty; it does not include small-to-large bowel anastomosis.
The risk of SSI in small bowel surgery is variable. The Surgical Site Infection Surveillance Service in England (data collected by 168 hospitals in 13 categories of surgical procedures between 1997 and 2002) reported an SSI rate of 8.9% (94 of 1056).393 Mean SSI rates for small bowel procedures reported in the most recent NHSN report (2006–08) were 3.44% for NHSN risk index category 0 versus 6.75% for NHSN risk index categories 1, 2, and 3. A study of 1472 patients undergoing bowel surgery (small bowel and colon) at 31 U.S. academic medical centers between September and December 2002 found an SSI rate of 8.7% for all wound categories. For patients with clean-contaminated wounds, the SSI rate was 7.9%; for those with contaminated or dirty-infected wounds, the SSI rates were 12.0% and 20.4%, respectively.394
In a study of 178 penetrating stomach and small bowel injuries, 94% of which were operated on within six hours of presentation, SSIs occurred in nearly 20% of cases. When associated colon injuries were excluded, SSIs occurred in 16% of gastric injuries and 13% of small bowel injuries. Although 74% of patients received antimicrobials, the specific timing of antimicrobial administration was not provided.395 Other studies of small bowel injury confirm similar SSI rates.396–400
Antimicrobial prophylaxis is recommended for small bowel surgery, based on inferring effectiveness from other clean-contaminated procedures. No specific prospective randomized studies could be identified that addressed antimicrobial prophylaxis for small bowel surgery. Antimicrobial prophylaxis for small bowel surgical procedures related to a diagnosis of complicated intra-abdominal infection is not addressed separately in these guidelines, as antimicrobial therapy for established intraabdominal infection should be initiated preoperatively.
Organisms. The most common microorganisms isolated from SSIs after small bowel surgery are aerobic gram-negative enteric organisms. Among the species isolated from patients with SSI after small intestine surgery are gram-negative bacilli of gastrointestinal enteric origin (aerobic and anaerobic) and gram-positive species, such as streptococci, staphylococci, and enterococci, which is consistent with similar studies.401 E. coli is the most frequently identified aerobe, indicating that the bowel flora constitute a major source of pathogens. Aerobic and anaerobic streptococci, Staphylococcus species, and Enterococcus species also have been reported.
The microbiology of 2280 SSIs after upper or lower abdominal surgery conducted from 1999 to 2006 was described in the Prevalence of Infections in Spanish Hospitals (EPINE) study.402 The most frequent microorganisms isolated were E. coli (28%), Enterococcus species (15%), Streptococcus species (8%), P. aeruginosa (7%), and S. aureus (5%; resistant to methicillin, 2%). The microbiology of SSIs after upper abdominal tract surgery did not show any significant differences compared with SSIs of the lower tract, though there were relatively more staphylococci, K. pneumoniae, Enterobacter species, Acinetobacter species, and Candida albicans isolates and fewer E. coli, B. fragilis, and Clostridium species in the upper abdominal surgery group.402
Efficacy. Antibiotic prophylaxis is generally recognized as effective in the prevention of postoperative SSIs in patients undergoing small bowel surgery when compared with placebo. However, there are no prospective placebo-controlled trials to definitively establish the efficacy of prophylactic antimicrobials in this patient population.
Choice of agent. The antimicrobials selected for prophylaxis must cover the expected pathogens for the small intestine. The microbial ecology of the proximal small intestine (i.e., jejunum) is similar to that of the duodenum, whereas the microbial flora of the ileum are similar to those of the colon. In patients with small intestine obstruction, the microbial flora are similar to those of the colon.
No randomized controlled trials have confirmed that one antimicrobial agent is superior to other agents for SSI prophylaxis in small bowel surgery. An appropriate antimicrobial choice for SSI prophylaxis in small bowel surgery is any single agent or combination of agents that provides adequate coverage for the small intestinal microbes. In patients with small bowel obstruction, additional coverage of anaerobic bacteria is also desirable.
For small intestine procedures with no evidence of obstruction, a first-generation cephalosporin (cefazolin) is recommended. For patients with small intestine obstruction, a first-generation cephalosporin with metronidazole or a second-generation cephalosporin with anaerobic activity (cefoxitin or cefotetan) is the recommended agent. The choice of agents should be based on local drug acquisition costs and antimicrobial sensitivity patterns. The Common Principles section of these guidelines provides additional considerations for weight-based dosing.
Duration. Preoperative dosing of antimicrobials for SSI prevention, with additional intraoperative antimicrobial dosing dependent on the duration of the operation and no postoperative dosing, is recommended for patients undergoing small bowel surgery.
Pediatric Efficacy. In pediatric patients, as with adults, antimicrobial prophylaxis for SSI prevention in small bowel surgery is recommended.
Recommendations. For small bowel surgery without obstruction, the recommended regimen is a first-generation cephalosporin (cefazolin) (Table 2). For small bowel surgery with intestinal obstruction, the recommended regimen is a cephalosporin with anaerobic activity (cefoxitin or cefotetan) or the combination of a first-generation cephalosporin (cefazolin) plus metronidazole. For β-lactam-allergic patients, alternative regimens include (1) clindamycin plus gentamicin, aztreonam, or a fluoroquinolone and (2) metronidazole plus gentamicin or a fluoroquinolone (ciprofloxacin or levofloxacin). (Strength of evidence for prophylaxis = C.)
Hernia Repair Procedures (Hernioplasty and Herniorrhaphy)
Background. All patients who undergo hernioplasty (prosthetic mesh repair of hernia) or herniorrhaphy (suture repair of hernia) should receive appropriate preoperative i.v. antimicrobials for SSI prevention. The risk of SSIs is higher in hernioplasty compared with herniorrhaphy.403 There is a significant risk of requiring prosthetic mesh removal in hernioplasty patients who develop an SSI, and determination of whether mesh placement will be required for hernia repair is not always possible in the preoperative period.
Mean SSI rates for herniorrhaphy reported in the most recent NHSN report (2006–08) were 0.74% (21 of 2852) for NHSN risk index category 0, 2.42% (81 of 3348) for NHSN risk index category 1, and 5.25% (67 of 1277) for NHSN risk index categories 2 and 3.165
A Cochrane meta-analysis of 17 randomized trials (n = 7843; 11 hernioplasty trials, 6 herniorrhaphy trials) in elective open inguinal hernia repair reported SSI rates of 3.1% versus 4.5% in the antimicrobial prophylaxis and control groups, respectively (OR, 0.64; 95% CI, 0.50–0.82).404 The subgroup of patients with herniorrhaphy had SSI rates of 3.5% and 4.9% in the prophylaxis and control groups, respectively (OR, 0.71; 95% CI, 0.51–1.00). The subgroup of patients with hernioplasty had SSI rates of 2.4% and 4.2% in the prophylaxis and control groups, respectively (OR, 0.56; 95% CI, 0.38–0.81).
A meta-analysis of nine randomized trials of open hernioplasty for inguinal hernia documented SSI rates of 2.4% (39 of 1642) in the antimicrobial group and 4.2% (70 of 1676) in the control group. Antibiotics showed a protective effect in preventing SSI after mesh inguinal hernia repair (OR, 0.61; 95% CI, 0.40–0.92). Antibiotic prophylaxis did reduce the rate of SSI in hernia patients undergoing mesh hernioplasty.405
Based on the results of these two systematic reviews, preoperative antimicrobial prophylaxis for SSI prevention is recommended for both herniorrhaphy and hernioplasty. Compared with open hernia repair, laparoscopic hernia repair has been reported to produce lower rates of incisional (superficial and deep) SSIs in randomized clinical trials.406–408 In a recent multicenter randomized trial of laparoscopic versus open ventral incisional hernia repair (n = 162), SSI was significantly less common in the laparoscopic group than in the open repair group (2.8% versus 21.9%; OR, 10.5; 95% CI, 2.3–48.2; p = 0.003).409 A meta-analysis of eight randomized trials comparing laparoscopic and open incisional or ventral hernia repair with mesh revealed that laparoscopic hernia repair was associated with decreased SSI rates (relative risk, 0.22; 95% CI, 0.09–0.54) and a trend toward fewer infections requiring mesh removal.410
Organisms. The most common microorganisms isolated from SSIs after herniorrhaphy and hernioplasty are aerobic gram-positive organisms. Aerobic streptococci, Staphylococcus species, and Enterococcus species are common, and MRSA is commonly found in prosthetic mesh infections.411
Efficacy. Antibiotic prophylaxis is generally recognized as effective when compared with placebo in the prevention of postoperative SSIs in patients undergoing herniorrhaphy and hernioplasty.
Choice of agent. Randomized controlled trials have failed to identify an agent that is clearly superior to other agents for SSI prophylaxis in hernia repair. A first-generation cephalosporin is the recommended agent on the basis of cost and tolerability. The Common Principles section of these guidelines provides additional considerations for weight-based dosing.
Duration. Based on the evidence to date, a single preoperative dose of antimicrobial is recommended in hernioplasty and herniorrhaphy, with redosing as recommended in the Common Principles section of these guidelines (if the procedure duration exceeds the recommended redosing interval from the time of initiation of the preoperative dose or if there is prolonged or excessive bleeding).
Recommendations. For hernioplasty and herniorrhaphy, the recommended regimen is a single dose of a first-generation cephalosporin (cefazolin) (Table 2). For patients known to be colonized with MRSA, it is reasonable to add a single preoperative dose of vancomycin to the recommended agent. For β-lactam-allergic patients, alternative regimens include clindamycin and vancomycin. (Strength of evidence for prophylaxis = A.)
Colorectal Procedures
Background. SSIs have been reported to occur in approximately 4–10% of patients undergoing colon procedures, 3–7% in small bowel procedures, and 3–27% in patients after rectal procedures, based on the risk index.165 However, when patients are followed carefully in clinical trials, rates tend to be considerably higher (17–26%).412 Other septic complications, such as fecal fistula, intra-abdominal abscesses, peritonitis, and septicemia, are serious concerns but are much less common.413 Infectious complication rates range from 30% to 60% without antimicrobial prophylaxis59,414 and are <10% with appropriate antimicrobial prophylaxis. A pooled analysis of clinical trials of antimicrobial prophylaxis in colon procedures demonstrated that antimicrobial use significantly reduced mortality rates (11.2% for control versus 4.5% for treatment) and SSI rates.415
The type and duration of the procedure can affect the risk of infection. Rectal resection is associated with a higher risk of infection than is intraperitoneal colon resection.416–418 Other risk factors include extended procedure duration (e.g., >3.5 hours),59,412,418,419 impaired host defenses,418 age of >60 years,418 hypoalbuminemia,419,420 bacterial or fecal contamination of the surgical site,418,420 inadvertent perforation or spillage,412,421 corticosteroid therapy,419 perioperative transfusion of packed red blood cells,394,418 hypothermia,422 hyperglycemia,423,424 and obesity.412,418
Organisms. The infecting organisms in colorectal procedures are derived from the bowel lumen, where there are high concentrations of organisms. B. fragilis and other obligate anaerobes are the most frequently isolated organisms from the bowel, with concentrations 1,000–10,000 times higher than those of aerobes.425 E. coli is the most common aerobe. B. fragilis and E. coli comprise approximately 20–30% of the fecal mass. They are the most frequently isolated pathogens from infected surgical sites after colon procedures.
Efficacy. Results from randomized controlled trials and a Cochrane review of 182 studies of over 30,000 patients support the routine use of prophylactic antimicrobials in all patients undergoing colorectal procedures.426
Choice of agent. The agent chosen for antimicrobial prophylaxis in colorectal procedures should have activity against the anaerobic and aerobic floras of the bowel. The most appropriate regimen for antimicrobial prophylaxis for colorectal procedure (e.g., oral, i.v, oral–i.v. combination) and the optimal choice of antimicrobial agent have not been fully resolved.
Oral regimens. The efficacy of oral prophylactic antimicrobial agents has been established in studies only when used with mechanical bowel preparation (MBP). A variety of oral agents administered after MBP have been evaluated for prophylaxis for colorectal procedures. The most common combinations include an aminoglycoside (neomycin and, less often, kanamycin, which is only available in injectable form in the United States) plus a medication with anaerobic activity, usually erythromycin427–434 or metronidazole.432,433,435–439 In placebo-controlled studies, the oral combination was significantly more effective than placebo in reducing SSIs.427,433,434,439,440 Postoperative SSI rates were 0–11% with neomycin plus erythromycin427–432 and 2–13% with neomycin and metronidazole.436–438 Combinations of neomycin and tetracycline,440 neomycin and clindamycin,436 and neomycin and tinidazole441 have also been used successfully, with postoperative SSI rates of <10%. The use of metronidazole as a single agent appears to be less effective, with reported SSI rates of 12–15%.442–444
Oral antimicrobials have been compared with i.v. agents in a few studies. Oral neomycin plus oral erythromycin was similarly effective as i.v. cefoxitin in one study429 but inferior in another445 and was similarly effective as i.v. ceftriaxone plus i.v. metronidazole in patients undergoing elective colorectal procedures.431 The addition of i.v. cefamandole to oral neomycin plus oral erythromycin did not improve efficacy.430 In one of these studies, oral neomycin and erythromycin were more effective than i.v. cefoxitin for procedures lasting longer than 4 hours.445 A randomized controlled study was stopped early due to the significantly higher rate of infection in the oral neomycin and erythromycin group (41%) compared with the single-dose i.v. metronidazole and ceftriaxone group (9.6%) (p < 0.01).446 Similarly, a study of oral metronidazole and kanamycin compared with the same medications given intravenously found an increased rate of postoperative sepsis (36% versus 6.5%, respectively) (p < 0.001), greater numbers of E. coli resistant to kanamycin, more bacterial overgrowth, and antimicrobial-associated pseudomembranous colitis in the oral group.447 However, the oral antimicrobials were not given on a schedule expected to be effective, as they were discontinued 36 hours before the procedure. The fact that oral antibiotics were given for three days rather than less than one day, as is the current practice, was suggested as a possible reason for the resistance and colitis observed.
I.V. regimens. A wide range of i.v. antimicrobials have been evaluated for prophylaxis in colorectal procedures. Cephalosporins are the most common agents, usually administered as a single agent. The majority of studies found that single-agent first-generation cephalosporins (cefazolin and cephalothin)445,448–451 were ineffective, with postoperative SSI rates ranging from 12% to 39%.448,449 The lack of efficacy is likely due to their lack of B. fragilis activity. The combination of cefazolin and metronidazole provides adequate coverage of pathogens and may be a cost-effective prophylaxis strategy.6,41
Second-generation cephalosporins with anaerobic activity, such as cefoxitin and cefotetan, have been widely evaluated. In single-agent therapy, SSI rates ranged from 0% to 17%91,417,445,452–459; however, more than half of the studies found SSI rates of >10%.
Third-generation agents, cefotaxime and ceftriaxone, have been evaluated in a few trials; postoperative SSI rates were 8–19% with single-agent use.456,460,461 In some studies, second- or third-generation cephalosporins were combined with other i.v. agents, most commonly metronidazole.452,459–462 However, in all but one of these studies, a combination of a second- or third-generation cephalosporin plus metronidazole was no more effective than the cephalosporin alone. The use of third- or fourth-generation cephalosporins for routine antimicrobial prophylaxis is not recommended as use may lead to development of resistant organisms.6,41,444,463 However, in institutions where there is increasing gram-negative resistance from isolates to first- and second-generation cephalosporins, a single dose of ceftriaxone plus metronidazole may be preferred over routine use of carbapenems.
Three small studies, with under 200 patients each, found i.v. ampicillin–sulbactam or amoxicillin–clavulanate to be as effective as i.v. combinations of gentamicin and metronidazole,464 gentamicin and clindamycin,465 and cefotaxime and metronidazole for preventing SSIs in elective colorectal procedures.
A randomized controlled study of adult patients undergoing elective colon or rectal procedures evaluated the use of a single high dose of gentamicin 4.5 mg/kg i.v. plus metronidazole 500 mg i.v. in sequential order over 30 minutes compared with multiple standard doses of gentamicin 1.5 mg/kg plus metronidazole given preoperatively and every 8 hours for 24 hours postoperatively.16 All patients underwent MBP before surgery. Patients with a serum creatinine concentration exceeding 1.7 mg/L were excluded from the study. No statistically significant differences were seen in deep and superficial incisional SSI rates between groups. Significantly fewer superficial SSIs were seen in the single-dose group compared with the multidose group in procedures lasting longer than 3.5 hours (22.2% versus 55%, p = 0.021). A pharmacodynamic study of these patients found the gentamicin concentration at the time of surgical-site closure as the strongest independent factor for infection.17 Of note, the infection rate was 80% in 10 patients with gentamicin concentrations of <0.5 mg/L.
Other i.v. agents that have been evaluated either alone or in combination include aminoglycosides,464,466–469 clindamycin,466 ampicillin,467,469–471 penicillins plus β-lactamase inhibitors,464,465,468,472,473 doxycycline,470,474–476 piperacillin,91,473 imipenem,462 and ciprofloxacin.300
Ertapenem, a broad-spectrum carbapenem, is approved by FDA for the prophylaxis of SSIs after elective colorectal procedures.67 Cefotetan is also FDA approved for surgical prophylaxis in clean-contaminated procedures (e.g., gastrointestinal procedures) in adult patients undergoing elective colon or rectal procedures.62 A large, multicenter, randomized controlled study compared a single 1-g i.v. dose of ertapenem with cefotetan 2 g i.v. infused within 60 minutes before surgical incision.412 All patients received MBP preoperatively. SSI rates were significantly lower in the ertapenem group versus cefotetan in the per-protocol (18.1% and 31.1%, respectively) and the modified intent-to-treat (17.1% and 50.9%) populations. Ertapenem was found to be superior to cefotetan for SSI prevention. Although not statistically significant, higher rates of skin-related events (i.e., pruritis and rash), gastrointestinal events, and C. difficile infection were seen in the ertapenem group. The study authors concluded that ertapenem is an acceptable alternative to cefotetan and cefoxitin. Routine use of ertapenem for surgical prophylaxis remains controversial due to theoretical concerns regarding increases in resistant organisms and a potential increase in adverse events.477
Alternative agents for patients with a high likelihood of past serious adverse event or allergy to β-lactams include (1) clindamycin plus an aminoglycoside, aztreonam, or a fluoroquinolone and (2) metronidazole plus an aminoglycoside or a fluoroquinolone.41
Combination oral and i.v. regimens. Combinations of oral and i.v. antimicrobials have been used in an attempt to further reduce postoperative infection rates. Regimens include oral neomycin and erythromycin plus i.v. administration of a cephalosporin,416,417,429,445,449,478,479 metronidazole,480,481 and gentamicin plus clindamycin.466 Postoperative SSI rates in these studies ranged from 0% to 7%. With one exception,416 there was no significant difference between oral neomycin–erythromycin plus an i.v. antimicrobial and oral neomycin–erythromycin alone.429,449,466,478 When combination oral and i.v. agents were compared with i.v. agents alone, combination therapy was favored in five of six studies417,429,445,449,480,482; the difference was significant in three.417,449,482 The most recent Cochrane review found that the infection rate was significantly lower with the combination of oral plus i.v. prophylaxis when compared with i.v. alone (relative risk, 0.55; p = 0.000084) or with oral prophylaxis alone (relative risk, 0.34; p = 0.024).426 A recent report of over 2000 patients recorded prospectively in the Michigan Surgical Quality Collaborative—Colectomy Best Practices Project and analyzed retrospectively revealed a significantly lower rate of postoperative infections when 370 colectomy patients received MBP and oral antimicrobial prophylaxis compared with propensity-matched patients receiving i.v. prophylaxis alone.483
A multicenter, randomized, controlled study of 491 patients who received MBP plus oral antimicrobials (kanamycin and erythromycin) with i.v. cefmetazole (not available in the United States but noted by the expert panel to have a similar spectrum of activity as cefotetan) or i.v. cefmetazole alone found no difference in SSI between groups for colon procedures.484 However, the combination of oral and i.v. antimicrobials was significantly better than i.v. alone for rectal procedures, particularly abdominoperineal excision. Another study found the postoperative SSI rates after rectal resection were 23% and 11%, respectively, for patients receiving i.v. cefoxitin and cefoxitin plus oral neomycin and erythromycin.417
The safety and tolerability of oral antimicrobials have been investigated in two studies. One case–control study found an increased incidence of C. difficile colitis among patients with oral plus i.v. antimicrobials and MBP compared with i.v. antimicrobials and MBP alone.485 However, another case–control study found a lower rate (not statistically significant) of C. difficile infection in patients who had received oral antimicrobials compared with those who had not (1.6% versus 2.9%, p = 0.09).486 A randomized controlled study of 300 patients undergoing elective colorectal procedures found significantly higher rates of nausea and vomiting among patients receiving three doses of oral antimicrobials (neomycin and metronidazole, 44% and 31%, respectively) in combination with i.v. cefoxitin and MBP compared with regimens including one dose of oral antimicrobials (18% and 11%, respectively) and no oral antimicrobials (13% and 9%, respectively).487 No difference was noted between groups for rates of abdominal pain, SSIs, or intraabdominal abscesses. An increased number of gastrointestinal adverse events was also reported in another comparative study in the combination oral and i.v. group (2.9%) compared with the i.v.-only group (2.1%), although the results were not statistically significant.484 Overall, the evidence suggests that the combination of oral antimicrobials with MBP in addition to i.v. prophylactic antimicrobials reduces the rate of postoperative infections compared with i.v. antimicrobials alone without MBP, although the addition of oral antimicrobials increases gastrointestinal symptoms.
Duration. Single and multiple doses were compared in several studies.454–456,461,471,475 However, only two of these studies compared single doses with multiple doses of the same antimicrobial.471,475 There was no significant difference in infection rates between single-dose and multidose administration. One study found a single dose of cefotaxime plus metronidazole was significantly more effective than three doses of cefotaxime alone.461 The most recent Cochrane review found no benefit to extending the duration of prophylaxis (p = 0.58).426 Generally, antimicrobial prophylaxis should be continued for no more than 24 hours and can typically be stopped when the procedure is completed and the surgical site is closed.6,41,444 No evidence supports greater efficacy for doses given after the completion of the procedure. Additional discussion on this topic is found in the Common Principles section of these guidelines.
Consideration should be given to an additional dose of the i.v. antimicrobial if an agent with a short half-life is used and the procedure duration exceeds the recommended redosing interval (starting from the time of initiation of the preoperative dose) and if intraoperative blood loss occurs.6,41,120,418,444,445 No significant difference was seen in SSI rates with single-dose cefazolin, single-dose cefotetan, and cefazolin given as one preoperative dose and a second dose three hours later for procedures with a duration of less than three hours.118 SSI rates were significantly higher with a single dose of cefazolin for procedures with a duration of greater than three hours. Using an agent with a longer half-life can decrease the necessity to redose the antimicrobial during long procedures.
Pediatric Efficacy. No well-controlled studies have evaluated the efficacy of antimicrobial prophylaxis in pediatric patients undergoing colorectal procedures. However, there is no reason to suspect that prophylaxis efficacy would be different. The safety, efficacy, tolerability, and cost-effectiveness of intestinal lavage have been demonstrated in two studies of 20 and 21 pediatric patients.488,489
Recommendations. A single dose of second-generation cephalosporin with both aerobic and anaerobic activities (cefoxitin or cefotetan) or cefazolin plus metronidazole is recommended for colon procedures (Table 2). In institutions where there is increasing resistance to first- and second-generation cephalosporins among gram-negative isolates from SSIs, the expert panel recommends a single dose of ceftriaxone plus metronidazole over routine use of carbapenems. An alternative regimen is ampicillin–sulbactam. In most patients, MBP combined with a combination of oral neomycin sulfate plus oral erythromycin base or oral neomycin sulfate plus oral metronidazole should be given in addition to i.v. prophylaxis. The oral antimicrobial should be given as three doses over approximately 10 hours the afternoon and evening before the operation and after the MBP. Alternative regimens for patients with β-lactam allergies include (1) clindamycin plus an aminoglycoside, aztreonam, or a fluoroquinolone and (2) metronidazole plus an aminoglycoside or a fluoroquinolone. Metronidazole plus aztreonam is not recommended as an alternative because this combination has no aerobic gram-positive activity.385 (Strength of evidence for prophylaxis = A.)
Head and Neck Procedures
Background. Elective procedures of the head and neck are predominantly clean or clean-contaminated.490 Clean procedures include thyroidectomy and lymph node excisions. Clean-contaminated procedures include all procedures involving an incision through the oral or pharyngeal mucosa, ranging from parotidectomy, submandibular gland excision, tonsillectomy, adenoidectomy, and rhinoplasty to complicated tumor-debulking and mandibular fracture repair procedures requiring reconstruction. The frequency of SSIs reported for clean procedures without antimicrobial prophylaxis is <1%.491,492 In contrast, infection rates in patients undergoing complicated head and neck cancer surgery are quite high, with infection occurring in 24–87% of patients without antimicrobial prophylaxis.493–497 While many of these head and neck cancer procedures are clean-contaminated, these procedures can fall into different wound classifications. Head and neck cancer patients often have many of the risk factors for infection mentioned below.498
Postoperative SSI rates are affected by age, nutritional status, and the presence of concomitant medical conditions such as diabetes mellitus, anemia, and peripheral vascular disease.496,499–504 Use of tobacco,498,505 alcohol,505,506 or drugs of abuse507 has also been associated with a higher risk of postoperative infection, particularly in patients with mandibular fracture. The hospital course, including length of hospitalization before operation, duration of antimicrobial use before operation, length of operation, presence of implants, and previous tracheotomy can also affect postoperative SSI rates.496,497,501–504,508 In patients with cancer, preoperative radiation and chemotherapy as well as the stage of the malignancy may also affect infection risk.497,498,502–504 Procedure-related risk factors for infection include radical or bilateral neck dissections501,508 and reconstruction with myocutaneous flaps or microvascular-free flaps.497–499,508
Organisms. The normal floras of the mouth and the oropharynx are responsible for most infections that follow clean-contaminated head and neck procedures.6,8,496,498,499,506,509–519 Anaerobic and aerobic bacteria are abundant in the oropharynx. As a result, postoperative SSIs are usually polymicrobial and involve both aerobic and anaerobic bacteria. The predominant oropharyngeal organisms include various streptococci (aerobic and anaerobic species), other oral anaerobes including Bacteroides species (but not B. fragilis), Peptostreptococcus species, Prevotella species, Fusobacterium species, Veillonella species, Enterobacteriaceae, and staphylococci. Nasal flora includes Staphylococcus species and Streptococcus species.
Efficacy. Clean procedures. Systemic administration of prophylactic antimicrobials has not been proven effective in reducing SSI rates in patients undergoing clean procedures of the head and neck and are not recommended for routine use.6–8,497,520 One randomized, double-blind, multicenter study of 500 patients undergoing thyroid procedures for goiter or carcinoma found no difference in postoperative SSI rates in those who received antimicrobial prophylaxis (0.8%) and those who did not (0.4%).491
Clean-contaminated procedures. Based on the best available evidence, current guidelines and review articles recommend the use of antimicrobial prophylaxis for the majority of clean-contaminated procedures.6–8,497,520,521 However, antimicrobial prophylaxis did not lower infection risk in randomized controlled trials of patients undergoing adenoidectomy, tonsillectomy,522,523 and septoplasty,524 and systematic reviews have not recommended prophylaxis for these procedures.7,525,526
The efficacy of antimicrobial prophylaxis is best established for head and neck cancer surgery. Several small randomized, controlled trials found high infection rates in placebo groups (24–78%) and markedly lower infection rates in the prophylaxis groups (5.8–38%) using a variety of regimens, including cefazolin, third-generation cephalosporins, and ampicillin plus cloxacillin. Although these studies were small, the results are concordant, and the high infection rates allowed the studies to reach statistical significance despite the small sample sizes. Similar results were reported in several additional small, uncontrolled studies.500,527–529
Choice of agent. Several randomized, single-center studies have compared antimicrobial regimens for clean-contaminated procedures. In one study, 189 patients undergoing head and neck cancer procedures were randomized to receive cefazolin 1 g (n = 92) or amoxicillin–clavulanate (n = 97), both given within one hour of incision and every eight hours postoperatively for three doses.511 The postoperative SSI rates were 24% with cefazolin and 21% with amoxicillin–clavulanate; there was no statistically significant difference in infection rates in this underpowered study. Two studies have compared ampicillin–sulbactam to clindamycin and yielded discordant results. One study of 242 patients (169 evaluable) undergoing head and neck cancer procedures compared ampicillin–sulbactam 1.5 g (n = 119) and clindamycin 600 mg (n = 123) given within one to two hours of incision and every six hours postoperatively for a total of four doses.510 No difference in SSIs was found, with 15 infections reported in each group (13% for the ampicillin–sulbactam group and 12% for the clindamycin group). There was no significant difference in adverse events between groups. There was a higher rate of C. difficile-positive patients in the clindamycin group (n = 7) than in the ampicillin–sulbactam group (n = 1), with no reported statistical analysis. Another study of 212 patients undergoing clean-contaminated head and neck oncology surgery found significantly fewer infections in the ampicillin–sulbactam group (13.3%) compared with the clindamycin group (27.1%) (p = 0.02).530 A greater number of gram-negative pathogens were recovered from patients randomized to the clindamycin group. The combination of gentamicin and clindamycin was superior to cefazolin in one older clinical trial.531
Duration. Studies of clean-contaminated head and neck procedures found no difference in efficacy between regimens of 24 hours and longer regimens of three, five, or seven days.499–501,505,507,512,524,531–534 Limited data exist on single-dose prophylaxis in these procedures.
One study of patients undergoing free-flap reconstruction after head and neck procedures found a significantly lower rate of acquisition and infection with MRSA in patients receiving short-term cefuroxime and metronidazole (one dose during induction of anesthesia and one dose eight hours postoperatively) compared with long-term therapy (same antimicrobials with additional doses every eight hours for up to five days) (p = 0.005 and p = 0.01, respectively, for acquisition and infection).535
Recommendations. Clean procedures. Antimicrobial prophylaxis is not required in patients undergoing clean surgical procedures of the head and neck. If there is placement of prosthetic material, a preoperative dose of cefazolin or cefuroxime is reasonable, though there are few data supporting the efficacy of prophylaxis in this setting (Table 2). A reasonable alternative for patients with β-lactam allergies is clindamycin. (Strength of evidence against prophylaxis without prosthesis placement = B; strength of evidence for prophylaxis with prosthesis placement = C.)
Clean-contaminated procedures. Antimicrobial prophylaxis has not been shown to benefit patients undergoing tonsillectomy or functional endoscopic sinus procedures. The preferred regimens for patients undergoing other clean-contaminated head and neck procedures are (1) cefazolin or cefuroxime plus metronidazole and (2) ampicillin–sulbactam. Clindamycin is a reasonable alternative in patients with a documented β-lactam allergy. The addition of an aminoglycoside to clindamycin may be appropriate when there is an increased likelihood of gram-negative contamination of the surgical site. (Strength of evidence for prophylaxis in cancer surgery patients = A; strength of evidence for prophylaxis for other clean-contaminated procedures except tonsillectomy and functional endoscopic sinus procedures = B.)
Neurosurgery Procedures
Background. Nosocomial central nervous system (CNS) infections do not often occur but have potentially serious consequences and poor outcomes, including death.536 One of the greatest risks for these infections in children and adults is undergoing a neurosurgical procedure. A classification system for neurosurgery, validated by Narotam et al.,537 divides procedures into five categories: clean, clean with foreign body, clean-contaminated, contaminated, and dirty. Risk factors for postoperative infections after neurological procedures include an ASA classification of ≥2,538 postoperative monitoring of intracranial pressure538,539 or ventricular drains536,538 for five or more days, cerebrospinal fluid (CSF) leak,539–541 procedure duration of more than two to four hours,540,542–544 diabetes,544 placement of foreign body,536 repeat or additional neurosurgical procedures,538,541–543 concurrent (remote, incision, or shunt) or previous shunt infection,536,539,545,546 and emergency procedures.542,545
Organisms. Data from most published clinical trials indicate that SSIs are primarily associated with gram-positive bacteria, S. aureus, and coagulase-negative staphylococci.6,8,537–545,547–554 Several cohort studies revealed high rates (up to 75–80% of isolates) of MRSA540–543,548–552 and coagulase-negative staphylococci among patients undergoing a variety of neurosurgical procedures.539,540,543,549 Other skin organisms such as P. acnes may be seen after CSF shunt placement, craniotomy, and other procedures.536,555,556 Gram-negative bacteria have also been isolated as the sole cause of postoperative neurosurgical SSIs in approximately 5–8% of cases and have been isolated in polymicrobial infections.537–539,541–545,547–550,552,553
Efficacy. Clean procedures. Antimicrobial prophylaxis is recommended for adult and pediatric patients undergoing craniotomy and spinal procedures.7,520 One meta-analysis of six studies found decreased odds of meningitis in patients undergoing craniotomy who received antimicrobial prophylaxis (1.1%) versus no prophylaxis (2.7%) (p = 0.03).557 Two cohort studies540,543 in patients undergoing craniotomy at the same institution found that antimicrobial prophylaxis with cloxacillin or amoxicillin–clavulanate, clindamycin for β-lactam-allergic patients, and other antimicrobials (not detailed) had a significantly lower infection rate (5.8%) than no prophylaxis (9.7%) (p < 0.0001).543 A significantly lower infection rate of 4.6% was seen in low-risk patients (clean craniotomy, no implant) with antimicrobial prophylaxis compared with those without prophylaxis (4.6% versus 10%, p < 0.0001). A significantly lower rate of scalp infections, bone flap osteitis, and abscess or empyema was seen with antimicrobial prophylaxis compared with no prophylaxis. Antimicrobial prophylaxis demonstrated no difference in postoperative meningitis540,543 and infection rates in high-risk patients (those undergoing emergency, clean-contaminated, and dirty procedures or reoperation or with operative times exceeding four hours).543
Prospective studies involving large numbers of patients have also demonstrated lower neurosurgical postoperative infection rates when antimicrobial prophylaxis is used.558–561 One such study of patients undergoing craniotomy, spinal, or shunting procedures was stopped early because of an excessive number of SSIs in the placebo group.562
Choice of agent. Studies of clean neurosurgical procedures reported antimicrobial regimens including clindamycin,540,543,557 vancomycin,542,557 cefotiam (not marketed in the United States),557 piperacillin,557 cloxacillin,540,543,557 oxacillin,542,557 cefuroxime,547 cefotaxime,548 sulfamethoxazole–trimethoprim,548 cefazolin,542,544 penicillin G,542 and amoxicillin–clavulanate.540,542,543 A meta-analysis found no significant difference in the rates of postcraniotomy meningitis with various antimicrobial regimens (single-dose regimens of clindamycin, vancomycin, or cefotiam; three doses of piperacillin; four doses of cloxacillin; and six doses of oxacillin).557
A randomized, open-label, multicenter study of 613 adult patients undergoing elective craniotomy, shunt, or stereotactic procedures found no difference in single doses of cefotaxime and trimethoprim–sulfamethoxazole in post-operative abscess formation, SSIs, and shunt infections.548
Duration. The majority of studies included single doses of antimicrobials; therefore, the use of single-dose antimicrobial prophylaxis given within 60 minutes before surgical incision in patients undergoing neurosurgery is generally recommened.6,7,520,540,543,547,548,557,563
Efficacy for CSF-Shunting Procedures. Antimicrobial prophylaxis is recommended for adults undergoing placement of a CSF shunt.7 Prophylaxis in patients undergoing ventriculostomy or intraventrical prophylaxis at the time of ventriculoperitoneal shunt insertion has shown some benefit in reducing infection but remains controversial due to limited evidence.6,7
Because CNS infections after shunting procedures are responsible for substantial mortality and morbidity, especially in children, the possible role of prophylactic antimicrobials in such procedures has been studied in numerous small, well-conducted, randomized controlled trials.564–571 Meticulous surgical and aseptic techniques and short procedure times were determined to be important factors in lowering infection rates after shunt placement. Although the number of patients studied in each trial was small, two meta-analyses of these data demonstrated that antimicrobial prophylaxis use in CSF-shunting procedures reduced the risk of infection by approximately 50%.572,573
Intrathecal pump placement involves the implantation of a permanent intrathecal catheter to allow instillation of medication. CNS infections may occur after these procedures, which are performed in both pediatric and adult populations. Several retrospective series have reported infection rates of 4.5–9% after intrathecal baclofen pump placement.574–576 There are minimal published trial data regarding appropriate prophylaxis for intrathecal pump procedures. It has been suggested that prophylaxis for intrathecal pump procedures be managed similarly to prophylaxis for CSF-shunting procedures.577
There is no consensus on the use of antimicrobial prophylaxis in patients with extraventricular drains (EVDs) or intracranial pressure monitors.134 An international survey of neurosurgeons and critical care medicine and infectious diseases specialists illustrates the difference in practices. The majority of neurosurgeons used or recommended the use of antimicrobial prophylaxis with EVDs (73.5%) and other monitoring devices (59%), compared with rates of 46–59% for critical care medicine specialists and 35% for infectious diseases specialists. The majority of specialists did not recommend or use antimicrobial-coated EVD catheters.
Two randomized controlled studies comparing antimicrobial-impregnated shunts to standard, non-antimicrobial-impregnated shunts along with antimicrobial prophylaxis with i.v. cephalosporin found a decrease in rates of shunt infections549 and a significant decrease in CSF infection with antimicrobial-impregnated shunts.545 At this time, routine use of antimicrobial-impregnated devices is not recommended; additional well-designed studies are needed to establish their place in therapy.7,578
Choice of agent. In CSF-shunting procedures, no single antimicrobial agent has been demonstrated to have greater efficacy than others.546,548,551–554,579 There is a lack of data on the necessity of antimicrobials with CNS penetration relating to prevention of infection in CNS shunting procedures.
Duration. The majority of studies support the use of single-dose prophylaxis regimens or regimens with a duration of 24–48 hours postoperatively.6–8,520,539,546,549–552,579 There is a lack of data evaluating the continuation of EVDs with and without antimicrobial prophylaxis. The international survey mentioned above asked respondents to indicate their recommended duration for antimicrobial prophylaxis with EVDs as either periprocedural, for 24 hours, for the first three days, for the entire time the device is in place, or other.135 The respondents from the specialties of neurosurgery, neurocritical care, and critical care had similar results, with 28–31% using or recommending periprocedural antimicrobials, 4–10% for 24 hours, 2–4% for the first three days, 43–64% for the entire time the device is in place, and 0–14% for other. The infectious diseases specialists reported rates of 62%, 19%, 4%, 12%, and 4%, respectively.
One retrospective single-center cohort study of 308 patients with EVDs placed for three days or more received antimicrobial prophylaxis for the duration of EVD use (n = 209) compared with patients receiving cefuroxime 1.5 g i.v. every eight hours for three doses or less frequently periprocedurally (timing not clearly defined in article) (n = 99).580 The overall rate of bacterial ventriculitis was 3.9%, with 8 patients (3.8%) in the extended-use group and 4 patients (4%) in the short-term prophylaxis group, the difference of which was not significant. The study authors concluded that there was no benefit to the use of a prolonged duration of antimicrobial prophylaxis.
Pediatric Efficacy for CSF-Shunting Procedures. Antimicrobial prophylaxis is recommended for children undergoing a CSF-shunting procedure.7 The efficacy of antimicrobial prophylaxis is extrapolated from adult studies.
A retrospective pediatric study of 384 CSF-shunting procedures found a lower infection rate in patients who received antimicrobials (2.1%) compared with those who did not (5.6%), but this difference failed to reach statistical significance.581 Two randomized, prospective studies that included pediatric patients did not demonstrate a significant difference in infection rates between the control group and the groups that received cefotiam571 (not available in the United States) or methicillin.568 A randomized, double-blind, placebo-controlled study that included pediatric patients undergoing ventriculoperitoneal shunt surgeries failed to demonstrate that the use of perioperative sulfamethoxazole–trimethoprim reduced the frequency of shunt infection.564
Other studies have demonstrated efficacy for prophylactic antimicrobials.566,582 A single-center, randomized, double-blind, placebo-controlled trial of perioperative rifampin plus trimethoprim was performed in pediatric patients.582 Among patients receiving rifampin plus trimethoprim, the infection rate was 12%, compared with 19% in patients receiving placebo. The study was ended because of the high infection rates before significance could be achieved. Infection rates at the study institution had been 7.5% in the years before the study. An open-label randomized study, including pediatric patients, demonstrated a lower infection rate in a group receiving oxacillin (3.3%) than in a control group (20%).566
Recommendations. A single dose of cefazolin is recommended for patients undergoing clean neurosurgical procedures, CSF-shunting procedures, or intrathecal pump placement (Table 2). Clindamycin or vancomycin should be reserved as an alternative agent for patients with a documented β-lactam allergy (vancomycin for MRSA-colonized patients). (Strength of evidence for prophylaxis = A.)
Cesarean Delivery Procedures
Background. Approximately 1.2 million infants are born by cesarean delivery in the United States annually.583 The infection rate after cesarean delivery has been reported to be 4–15%,583 though recent NHSN data showed an infection rate of 2–4%.165
Postpartum infectious complications are common after cesarean delivery. Endometritis (infection of the uterine lining) is usually identified by fever, malaise, tachycardia, abdominal pain, uterine tenderness, and sometimes abnormal or foul-smelling lochia.584 Fever may also be the only symptom of endometritis.
Endometritis has been reported to occur in up to 24% of patients in elective cesarean delivery and up to approximately 60% of patients undergoing nonelective or emergency section.584,585 Risk factors for endometritis include cesarean delivery, prolonged rupture of membranes, prolonged labor with multiple vaginal examinations, intrapartum fever, and low socioeconomic status.585,586 Patients with low socioeconomic status may have received inadequate prenatal care.
The factor most frequently associated with infectious morbidity in postcesarean delivery is prolonged labor in the presence of ruptured membranes. Intact chorioamniotic membranes serve as a protective barrier against bacterial infection. Rupture of the membrane exposes the uterine surface to bacteria from the birth canal. The vaginal fluid with bacterial flora is drawn into the uterus when it relaxes between contractions during labor. Women undergoing labor for more than six to eight hours in the presence of ruptured membranes should be considered at high risk for developing endometritis.587 Other risk factors for SSIs after cesarean delivery include systemic illness, poor hygiene, obesity, and anemia.587,588
Organisms. The normal flora of the vagina include staphylococci, streptococci, enterococci, lactobacilli, diphtheroids, E. coli, anaerobic streptococci (Peptococcus species and Peptostreptococcus species), Bacteroides species (e.g., Bacteroides bivius, B. fragilis), and Fusobacterium species.584,587,589–592 Endometritis infections are often polymicrobial and include aerobic streptococcus (particularly group B β-hemolytic streptococcus and enterococci), gram-negative aerobes (particularly E. coli), gram-negative anaerobic rods (particularly B. bivius), and anaerobic cocci (Peptococcus species and Peptostreptococcus species). Ureaplasma urealyticum has been commonly isolated from endometrial and surgical-site cultures. Additional commonly isolated organisms from SSIs include Staphylococcus species and enterococci.
Efficacy. While the use of antimicrobial prophylaxis in low-risk procedures (i.e., those with no active labor and no rupture of membranes) has been brought into question by the results of several randomized, placebo-controlled studies that found no reduction in infectious complications (fever, SSI, urinary tract infection, or endometritis) with the use of prophylaxis, the majority of these evaluations were underpowered and included administration of antimicrobial prophylaxis at cord clamping.593–599 However, the efficacy of antimicrobial prophylaxis in cesarean delivery has been shown in several studies and two meta-analyses for both elective and nonelective procedures. Therefore, prophylaxis is recommended for all patients undergoing cesarean delivery.584,592
One meta-analysis that reviewed 7 placebo-controlled randomized trials in low-risk elective cesarean delivery found that prophylaxis was associated with a significant decrease in endometritis and fever.592 A larger meta-analysis of 81 randomized trials with 11,937 women undergoing both elective and nonelective cesarean delivery found that antimicrobial prophylaxis was associated with a significant reduction in risk of fever, endometritis, SSI, urinary tract infection, and serious infection.585 The relative risk for endometritis in elective cesarean section was 0.38 (95% CI, 0.22–0.64) in those receiving antimicrobial prophylaxis compared to those receiving no prophylaxis.
Choice of agent. Although several different antimicrobials used alone or in combination for antimicrobial prophylaxis during cesarean delivery have been evaluated, the use of first-generation cephalosporins (specifically cefazolin) has been advocated by ACOG and the American Academy of Pediatrics (AAP), based on their efficacy, narrow spectrum of activity, and low cost.584 This recommendation is supported by a meta-analysis of 51 randomized controlled trials comparing at least two antimicrobial regimens that concluded that ampicillin and first-generation cephalosporins have similar efficacy.600
Newer prospective randomized controlled and cohort studies have evaluated the addition of metronidazole, azithromycin,601–603 or doxycycline601 to a first- or second-generation cephalosporin to extend the spectrum of activity against common organisms isolated from endometrial and surgical-site cultures, specifically U. urealyticum and Mycoplasma species. These studies found significantly lower rates of postoperative infections (including endometritis and SSI) and a shorter duration of hospital stay compared with prophylaxis with a first- or second-generation cephalosporin alone.601–604 Antibiotic administration occurred either postoperatively or after cord clamping in these studies. Further study, particularly with preoperative antimicrobial administration, is needed to confirm these preliminary findings and establish a place in therapy for this practice.
Timing. Historically, administration of antimicrobials in cesarean delivery was delayed until after cord clamping.600,605,606 The principal reasons were to avoid suppression of the neonate’s normal bacterial flora that could promote the selection of resistant organisms and concern that the antimicrobials could potentially mask neonatal infection, complicating evaluation of neonatal sepsis. However, more contemporary data support the administration of antimicrobial prophylaxis before surgical incision to protect against bacterial contamination of the surgical site and decrease the risk of infection. The practice of antimicrobial prophylaxis administration before surgical incision is endorsed by ACOG and AAP.584,607 See the Common Principles section of these guidelines for additional discussion on antimicrobial timing.
A meta-analysis of three randomized controlled trials and two nonrandomized controlled studies provided evidence that preoperative antimicrobial administration significantly decreased the rate of endometritis compared with administration after cord clamping (3.9% and 8.9%, respectively; p = 0.012).605 A lower SSI rate was also seen with preoperative antimicrobial administration (3.2% versus 5.4%), though this difference was not significant. The overall rate of infection-related morbidity was also significantly lower. No differences between the groups were seen in neonatal outcomes, including sepsis, sepsis workups, and neonatal intensive care unit admissions. The largest study included in this meta-analysis was a prospective, randomized, controlled, double-blind, single-center, double-dummy study of 357 patients comparing cefazolin 1 g i.v. given preoperatively and after cord clamping, which had results consistent with the overall meta-analysis.606
In a recent randomized trial of more than 1100 women undergoing cesarean section between 2004 and 2010, Witt and colleagues608 found no difference in SSI rates for patients having antimicrobial administration before surgical incision compared with those who received antimicrobial prophylaxis at the time of cord clamping. All patients received a single dose of cefazolin 2 g.
Duration. A meta-analysis of 51 studies found that multidose regimens provided no apparent benefit over single-dose regimens.600 The use of single-dose prophylaxis is supported by ACOG and AAP for procedures lasting less than two hours.584 Additional intraoperative doses may be warranted for patients with excessive blood loss or for whom the duration of the procedure is extended.
For additional discussion of dosing, see the Common Principles section of these guidelines.
Recommendation. The recommended regimen for all women undergoing cesarean delivery is a single dose of cefazolin administered before surgical incision (Table 2). (Strength of evidence for prophylaxis = A.) For patients with β-lactam allergies, an alternative regimen is clindamycin plus gentamicin.
Hysterectomy Procedures
Background. Hysterectomy is second only to cesarean delivery as the most frequently performed major gynecological procedure in the United States, with over 600,000 hysterectomies performed annually.609 Uterine fibroid tumors account for 40% of all presurgical diagnoses leading to hysterectomy.609 Other common diagnoses are dysfunctional uterine bleeding, genital prolapse, endometriosis, chronic pelvic pain, pelvic inflammatory disease, endometrial hyperplasia, and cancer.
Hysterectomy involves the removal of the uterus and, occasionally, one or two fallopian tubes, the ovaries, or a combination of ovaries and fallopian tubes.610 Radical hysterectomy entails removal of the uterus, fallopian tubes, and ovaries and extensive stripping of the pelvic lymph nodes in patients with extension of their cancer. Hysterectomies are performed by a vaginal or abdominal approach using a laparoscopic- or robot-assisted method. During a vaginal hysterectomy, the procedure is completed through the vagina with no abdominal incision. Abdominal hysterectomy involves an abdominal incision. Laparoscopic and robotic methods involve small incisions and require additional equipment, increased operator experience, and increased length of procedures.611,612 In the United States, between 2000 and 2004, the abdominal approach for hysterectomy was used in 67.9% of surgical procedures and the vaginal approach in 32.1%. Of hysterectomies performed via the vaginal approach, 32.4% also used laparoscopy.609 The ACOG Committee on Gynecologic Practice recommends vaginal hysterectomy as the approach of choice for benign disease, based on evidence of better outcomes and fewer complications.613 Laparoscopic abdominal hysterectomy is an alternative when the vaginal route is not indicated or feasible.613,614 Of note, ACOG has stated that the supracervical approach—removal of the uterus with preservation of the cervix—should not be recommended as a superior technique for hysterectomy due to the lack of advantage in postoperative complications, urinary symptoms, or sexual function and the increased risk of future trachelectomy to remove the cervical stump.615
Infections after hysterectomy include superficial and organ/space (vaginal cuff infection, pelvic cellulitis, and pelvic abscess) SSIs.589 The reported SSI rates between January 2006 and December 2008 in the United States, based on NNIS risk index category, were 0.73–1.16 per 100 procedures for vaginal hysterectomy and 1.10–4.05 per 100 procedures for abdominal hysterectomy.165 A multicenter surveillance study found a mean infection rate of 2.53% associated with all types of hysterectomy and a significantly lower mean rate of infection with laparoscopic versus abdominal hysterectomies (1.15% versus 3.44%, respectively).325
Risk factors for infection after vaginal or abdominal hysterectomy include longer duration of surgery, young age, diabetes, obesity, peripheral vascular disease, collagen disease, anemia, transfusion, poor nutritional status, and previous history of postsurgical infection.590,616–622 The depth of subcutaneous tissue is also a significant risk factor for infection after abdominal hysterectomy.623 Additional risk factors for infection after radical hysterectomy for cervical cancer include the presence of malignancy, prior radiation therapy, and the presence of indwelling drainage catheters.619,620
Organisms. The vagina is normally colonized with a wide variety of bacteria, including gram-positive and gram-negative aerobes and anaerobes. The normal flora of the vagina includes staphylococci, streptococci, enterococci, lactobacilli, diphtheroids, E. coli, anaerobic streptococci, Bacteroides species, and Fusobacterium species.589,624 Postoperative vaginal flora differs from preoperative flora; the amount of enterococci, gram-negative bacilli, and Bacteroides species increases postoperatively. Postoperative changes in flora may occur independently of prophylactic antimicrobial administration and are not by themselves predictive of postoperative infection.589,625,626 Postoperative infections associated with vaginal hysterectomy are frequently polymicrobial, with enterococci, aerobic gram-negative bacilli, and Bacteroides species isolated most frequently. Postoperative SSIs after abdominal and radical hysterectomies are also polymicrobial; gram-positive cocci and enteric gram-negative bacilli predominate, and anaerobes are frequently isolated.626,627
Efficacy. A meta-analysis of 25 randomized controlled trials demonstrated the efficacy of antimicrobial prophylaxis, including first- and second-generation cephalosporins and metronidazole, in the prevention of infections after abdominal hysterectomy.628 The infection rates were 21.1% with placebo or no prophylaxis and 9.0% with any antimicrobial. Another meta-analysis found that the rate of postoperative infection (surgical and pelvic sites) in women undergoing vaginal hysterectomy who received placebo or no prophylactic antimicrobial ranged from 14% to 57%, which was significantly higher than the 10% rate reported with antimicrobials.629
Malignant disease as the reason for hysterectomy is a common exclusion from studies of antimicrobial prophylaxis. Older, prospective, placebo-controlled studies found a lower rate of SSIs with antimicrobial prophylaxis after radical hysterectomy.619,630–633 The applicability of these results is limited by small sample size and the inclusion of antimicrobials not available in the United States. Radical hysterectomy is primarily completed through an abdominal approach but can also be performed by a vaginal approach and using laparoscopic or robotic methods.634 Therefore, antimicrobial prophylaxis would be warranted, regardless of approach. No placebo-controlled studies have been conducted to evaluate the efficacy of antimicrobial prophylaxis when used for laparoscopic hysterectomy.
Choice of agent. Cephalosporins are the most frequently used and studied antimicrobials for prophylaxis in vaginal and abdominal hysterectomies. Studies directly comparing different cephalosporins have found no significant differences in rates of infection in vaginal hysterectomy and have indicated that first-generation cephalosporins (primarily cefazolin) are equivalent to second- and third-generation agents.635–644 In abdominal hysterectomy, no significant differences in the rates of serious infections were noted between second- and third-generation cephalosporin regimens.641,645–649 Few comparisons have been made between second-generation cephalosporins and cefazolin. Cefazolin has been at least as effective in preventing infectious complications as second- and third-generation cephalosporins.636,650–652 However, one double-blind controlled study of 511 women undergoing abdominal hysterectomy found that the risk of major SSIs requiring antimicrobial therapy was significantly higher in the group receiving preoperative cefazolin 1 g (11.6%; relative risk, 1.84; 95% CI, 1.03–3.29) than in those treated with cefotetan 1 g (6.3%).617 A multicenter, randomized, double-blind, active- and placebo-controlled study compared single doses of ampicillin, cefazolin, and placebo administered to women undergoing elective total abdominal hysterectomy at two centers in Thailand.653 The study found a significantly lower rate of infection, including superficial and deep SSIs, urinary tract infections, vaginal cuff infection, and pneumonia, with cefazolin (10.3%) compared with placebo (26.9%) and ampicillin (22.6%). No difference was seen between ampicillin and placebo. The study authors concluded that cefazolin was more effective than ampicillin for elective total abdominal hysterectomy.
A randomized controlled study of 511 patients undergoing laparoscopic gynecological procedures at one center in Italy compared single doses of amoxicillin–clavulanate 2.2 g and cefazolin 2 g i.v. administered 20–30 minutes before the procedure.654 A second dose was given if the surgery lasted over three hours or there was extensive blood loss (>1500 mL). No significant differences in the rates of any postoperative infection, including SSIs, were found between groups. The statistical power of the study was not stated.
In light of the organisms encountered in the vaginal canal and comparative studies conducted among different classes of cephalosporins, cefazolin, cefotetan, cefoxitin, cefuroxime, and ampicillin–sulbactam have been supported as appropriate first-line choices for prophylaxis during vaginal or abdominal hysterectomy.6,9,41 Alternative agents for patients with a history of immediate hypersensitivity to penicillin include either clindamycin or metronidazole plus an aminoglycoside or a fluoroquinolone (ciprofloxacin, levofloxacin, or moxifloxacin) or aztreonam (with clindamycin only).
Duration. Studies comparing single doses of one antimicrobial with multidose regimens of a different antimicrobial have shown the two regimens to be equally effective in reducing the postoperative infection rate in women undergoing vaginal and abdominal hysterectomies.635–643,645–650,655–663 The limited comparative trials involving single-dose cefazolin637,654,655,664 or ampicillin–sulbactam654,663 indicate that a single dose of antimicrobial is sufficient prophylaxis for SSIs for vaginal hysterectomy. Single doses of cefotetan, ceftizoxime, or cefotaxime appear to be as effective as multiple doses of cefoxitin.644–649,665 A second dose of antimicrobial is warranted when the procedure lasts three hours or longer or if blood loss exceeds 1500 mL.9,654
Recommendation. The recommended regimen for women undergoing vaginal or abdominal hysterectomy, using an open or laparoscopic approach, is a single dose of cefazolin (Table 2). Cefoxitin, cefotetan, or ampicillin–sulbactam may also be used. Alternative agents for patients with a β-lactam allergy include (1) either clindamycin or vancomycin plus an aminoglycoside, aztreonam, or a fluoroquinolone and (2) metronidazole plus an aminoglycoside or a fluoroquinolone. (Strength of evidence for prophylaxis = A.)
Ophthalmic Procedures
Background. Ophthalmic procedures include cataract extractions, vitrectomies, keratoplasties, intraocular lens implantation, glaucoma procedures, strabotomies, retinal detachment repair, laser in situ keratomileusis, and laser-assisted subepithelial keratectomy. Most of the available data regarding antimicrobial prophylaxis involve cataract procedures. The goal of prophylaxis is primarily to reduce acute postoperative endophthalmitis, defined as severe intraocular inflammation due to infection, which can lead to loss of vision if untreated.666 Since 2000, the reported frequency of endophthalmitis after ophthalmic procedures is low worldwide, ranging from 0% to 0.63%.667–680 The reported time from procedure to diagnosis of endophthalmitis ranges from one day to six weeks, with the majority of infections identified within one week.666,669,671,673,674,681–683
Potential risk factors for postoperative ophthalmic infections include preoperative factors such as diabetes,666 active ocular infection or colonization,666,684 lacrimal drainage system infection or obstruction, age of >85 years,685 and immunodeficiency.684 Procedure-related risk factors include clear corneal incisions (as opposed to scleral tunnel incisions),680,686 any surgical complication, vitreous loss,684 posterior capsule tear,681,684,685 silicone intraocular lens implantation,677,680 and the nonuse of facemasks in the operating theater.681
Organisms. Among organisms isolated from patients developing postoperative endophthalmitis after cataract procedure, approximately 25–60% were coagulase-negative Staphylococcus species, primarily S. epidermidis.668,670,671,673,674,678,683,684,686 Other gram-positive organisms identified included S. aureus, Streptococcus species, Enterococcus species, P. acnes, and Corynebacterium species. Gram-negative organisms isolated included Serratia species, Klebsiella species, P. mirabilis, and P. aeruginosa. These organisms represent the normal flora isolated pre-operatively in a number of studies.675,687–693
Efficacy. Data on antimicrobial prophylaxis efficacy in ophthalmic procedures to prevent endophthalmitis are limited; however, prophylaxis is common.684 The low rate of postoperative endophthalmitis makes it difficult to complete an adequately powered study to show efficacy of antimicrobial prophylaxis in ophthalmic procedures; therefore, surrogate markers of eradication of normal flora bacteria and reduction of bacterial count on the conjunctiva, lower and upper eyelids, eyelashes, and inner canthus (corner of the eye) preoperatively and postoperatively are used. Many of the available studies are flawed with retrospective or uncontrolled design, inadequate follow-up, variations in surgical techniques (including disinfection, antimicrobial prophylaxis strategies, and methods for performing procedures), and limited reporting of clinical outcomes.
The large, randomized, partially-masked, placebo-controlled, multinational, multicenter study conducted by the European Society of Cataract and Refractive Surgeons (ESCRS) compared the rate of postoperative endophthalmitis in over 16,600 patients undergoing routine cataract procedures at 24 centers in Europe randomized to one of four perioperative prophylaxis groups.679,680,694 Patients received no antimicrobial prophylaxis, intracameral cefuroxime at the end of the procedure alone, perioperative levofloxacin 0.5% ophthalmic solution given within the hour before the procedure, or both intracameral cefuroxime and perioperative levofloxacin. All patients had the eye area disinfected with povidone–iodine 5% preoperatively and received topical levofloxacin postoperatively. The study was stopped after an interim analysis due to results of a multivariate analysis indicating that patients not receiving intracameral cefuroxime were approximately five times more likely to develop endophthalmitis. The study has been questioned for its high rate of endophthalmitis, selection of cefuroxime due to gaps in gram-negative coverage, unknown drug concentrations in the aqueous humor, risks of hypersensitivity, the lack of a commercially available preparation, the lack of a subconjunctival cefuroxime treatment group, selection of topical levofloxacin, and methods for statistical analysis.695–697
Two single-center, historical-controlled studies in hospitals in Spain reported decreases in acute postoperative endophthalmitis among patients undergoing cataract procedure with intracameral cefazolin added to the previous routine prophylaxis of preoperative eyelid cleansing with soap for three days670 and povidone–iodine eye area preparation,670,674
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree