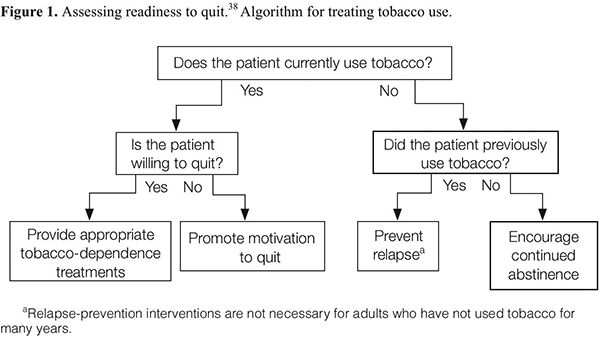
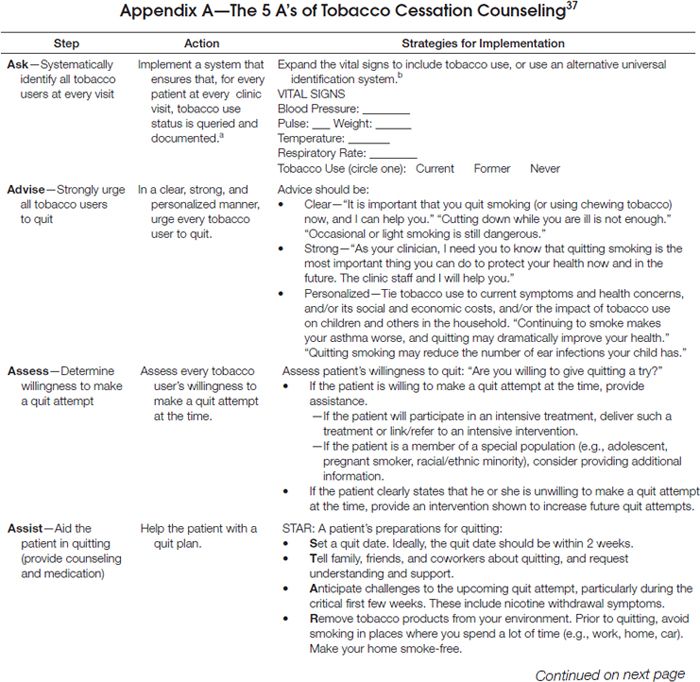
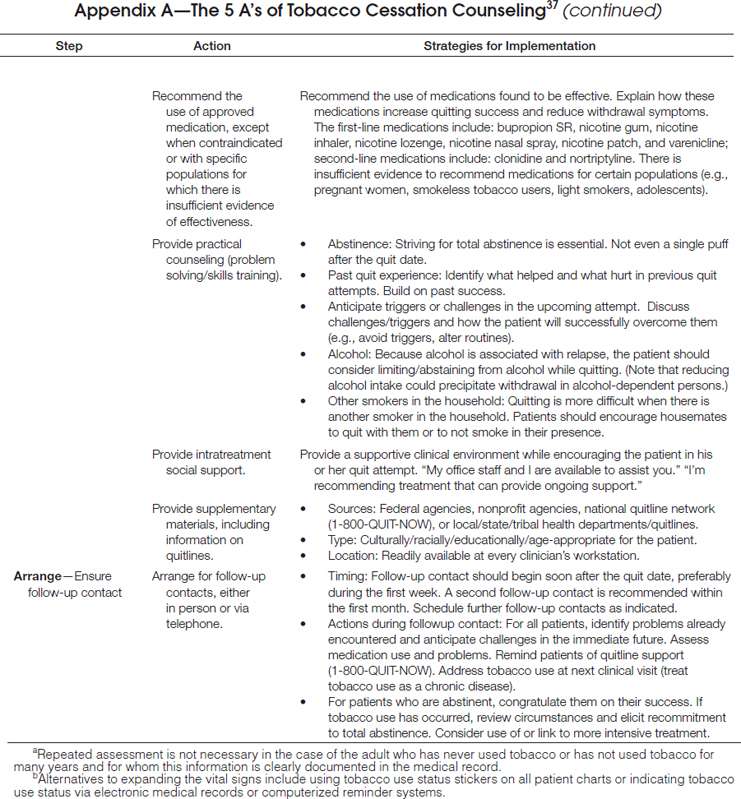
As delineated in the clinical practice guideline, clinicians should routinely screen for tobacco use and apply appropriate interventions (Figure 1), addressing five key components for comprehensive tobacco-cessation counseling (the 5 A’s): (1) asking patients about tobacco use, (2) advising tobacco users to quit, (3) assessing their willingness to make a quit attempt, (4) assisting patients with quitting, and (5) arranging follow-up care (Appendix A). To increase the odds for success, patients who are not ready to quit should receive tailored motivational interventions that address the 5 R’s37 (Appendix B). Patients who are ready to quit should be provided with a treatment plan that includes behavioral counseling plus pharmacotherapy (as appropriate) and follow-up counseling.
Clinicians should become familiar with local community-based resources for tobacco cessation, including group programs and telephone counseling.37 When expertise, practice site logistics, or time constraints do not afford an opportunity to provide comprehensive tobacco-cessation counseling, clinicians should apply a truncated 5 A’s model whereby they ask about tobacco use, advise tobacco users to quit, and then refer patients to appropriate cessation providers or programs. With the October 2004 introduction of a national toll-free quit line number (1–800-QUIT-NOW), all residents of the United States can receive tobacco-cessation counseling at no cost. In clinical trials, telephone counseling services for smoking cessation have been shown to be effective among the patients who use them.37–39 These positive results have been shown to translate into real-world effectiveness,40 as evidenced in several meta-analytic reviews.37,38,41,42
Even the busiest of clinicians can serve an important role by spending approximately one minute per patient to identify tobacco users and to provide referrals to the quit line for more comprehensive counseling. When patients indicate a willingness to set a quit date in the next month, clinicians should consider a proactive approach whereby they obtain the patient’s permission to contact the quit line directly on the patient’s behalf as opposed to their providing a passive referral (by simply offering the patient contact information for the quit line).38
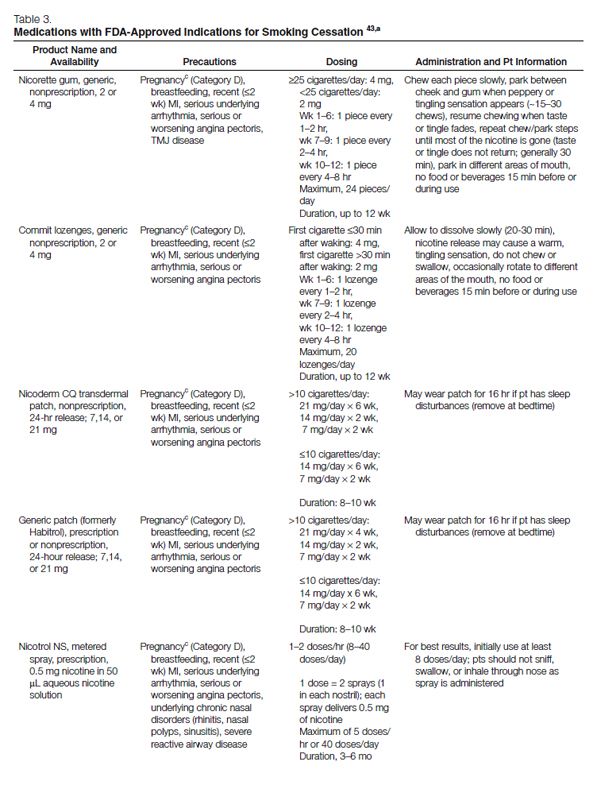
Pharmacotherapy for Cessation. All patients attempting to quit using tobacco should be encouraged to use effective pharmacotherapies (Table 3) for smoking cessation,43 except when medically contraindicated or in specific populations in which there is insufficient evidence of effectiveness (e.g., pregnant women, smokeless tobacco users, light smokers, adolescents).37 Currently, the agents with approved FDA labeling for smoking cessation include five nicotine-replacement therapy (NRT) dosage forms, sustained-release bupropion, and varenicline. Pharmacologic agents that have not received FDA approval for use in smoking cessation but have demonstrated efficacy are clonidine and nortriptyline.37
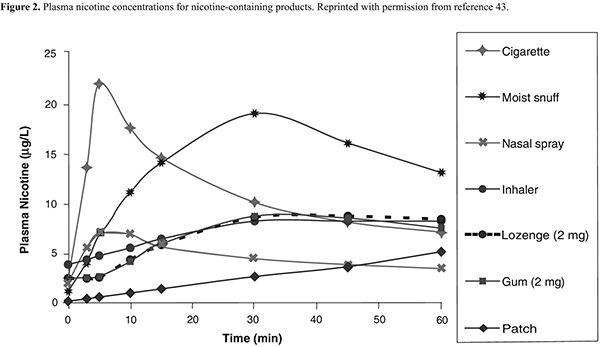
NRT. Formulations of NRT products currently available in the United States include nicotine gum, lozenge, transdermal patch, nasal spray, and oral inhaler (Table 3). These agents improve quit rates by reducing the symptoms of nicotine withdrawal, enabling the patient to focus on behavior modification and coping with the psychological aspects of quitting. In addition, because the onset of action with NRT is not as rapid as that of nicotine obtained through tobacco (Figure 2), patients become less accustomed to the nearly immediate, reinforcing effects of tobacco. Patients using NRT are approximately two times as likely to quit smoking than are those receiving placebo.37
For selected patients with cardiovascular disease,37 NRT should be used with caution, because nicotine can increase the heart rate and blood pressure and also act as a coronary vasoconstrictor.44 Despite these effects, randomized, controlled trials have found no significant increase in the incidence of cardiovascular events or mortality among patients with cardiovascular disease receiving NRT when compared with that of patients receiving placebo.45–47 However, because these studies specifically excluded patients with severe, underlying cardiac diseases, the clinical practice guideline37 recommends that because of a lack of safety data in these higher-risk populations, NRT should be used with caution among patients who have experienced a myocardial infarction within the past two weeks and among those with serious arrhythmias or unstable angina.37 A large observational study of more than 33,000 patients found that NRT use was not associated with an increased risk of myocardial infarction, stroke, or death.48 Because the serum concentrations of nicotine achieved with the recommended dosages of NRT are generally much lower than those attained with smoking, most experts have concluded that the risks associated with NRT use in patients with cardiovascular disease are minimal relative to the risks of continued tobacco use.44,49–52
Other conditions for which NRT should be used with caution include active temporomandibular joint disease, pregnancy, and lactation. Patients with active temporomandibular joint disease should not use nicotine gum because doing so might exacerbate their condition. FDA classifies prescription formulations of nicotine as pregnancy category D, indicating that there is evidence of risk to the human fetus. Accordingly, none of the NRT formulations have received FDA approval for use during pregnancy. Although NRT may pose a risk to the developing fetus, it is arguably less than the risks associated with continued smoking.53 However, because NRT has the potential to cause fetal harm and because of insufficient evidence supporting its efficacy in pregnant women, the 2008 clinical practice guideline does not recommend use during pregnancy.37 Nicotine is secreted in breast milk53 and, while the risk is slight, clinicians should be aware that the nursing infant is at risk for nicotine toxicity, especially if the mother concomitantly smokes and uses NRT.
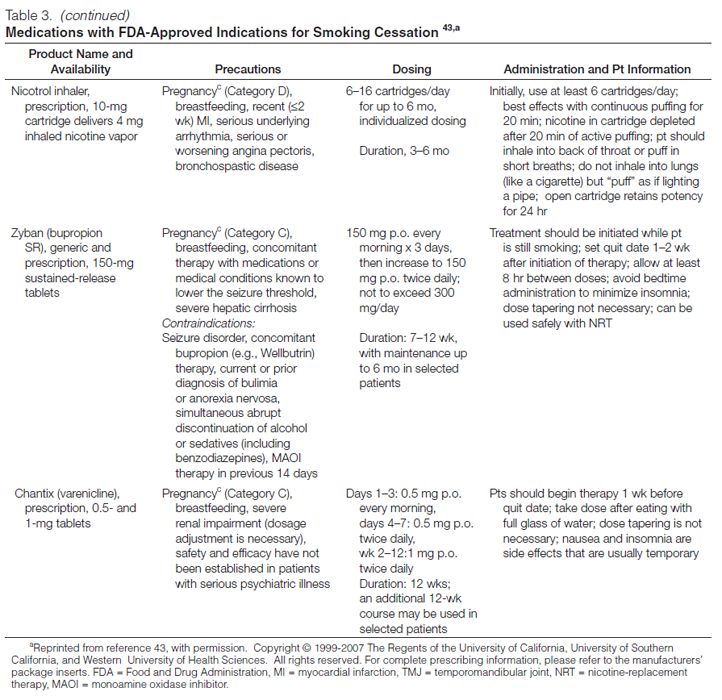
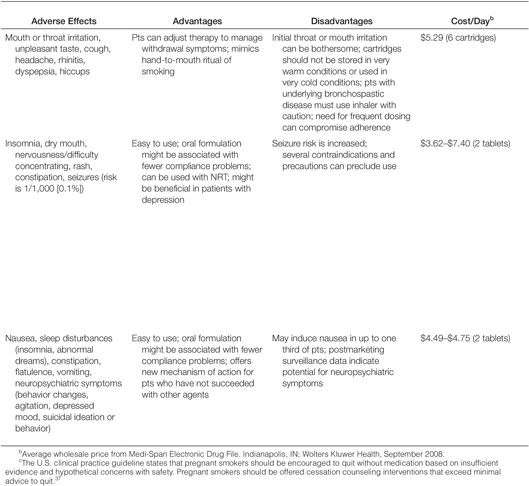
Sustained-Release Bupropion. The first nonnicotine medication FDA approved for smoking cessation was sustained-release bupropion (Zyban, GlaxoSmithKline, Philadelphia). This agent, which was originally marketed as an antidepressant, is hypothesized to promote smoking cessation by inhibiting the reuptake of dopamine and norepinephrine in the central nervous system54 and may function as a nicotinic acetylcholine-receptor antagonist.55 The neurochemical effects are believed to modulate the dopamine reward pathway and reduce the cravings for nicotine and symptoms of withdrawal.37 Bupropion is effective in smokers with or without a history of major depression,56 suggesting that the mechanism of action is independent of the agent’s antidepressant effects. Clinical trials involving almost 10,000 participants have confirmed the effectiveness of sustained-release bupropion as an aid to tobacco cessation; a recent meta-analysis of 31 trials concluded that the odds of tobacco abstinence at six or more months was 1.9 (sustained-release bupropion relative to placebo, 95% CI, 1.7–2.2).57 Patients receiving sustained-release bupropion are approximately two times more likely to quit smoking than are patients receiving placebo.44
Bupropion is contraindicated in patients (1) with a seizure disorder, (2) with a history of anorexia or bulimia nervosa, (3) who are using another formulation of bupropion (Wellbutrin, Wellbutrin SR, Wellbutrin XL), (4) who have used a monoamine oxidase inhibitor within the past 14 days, and (5) who are undergoing abrupt discontinuation of alcohol or sedatives (including benzodiazepines).58 ln clinical trials for smoking cessation, the frequency of seizures with bupropion was <0.1% (seven seizures among 8000 bupropion-treated patients).57 This frequency is comparable to the report rate of seizures (0.1%) when sustained-release bupropion was used in the treatment of depression.59 For this reason, bupropion should be used with extreme caution in patients with a history of seizures, those who have experienced cranial trauma, and those receiving medications known to lower the seizure threshold or those with underlying severe hepatic cirrhosis. In addition, caution should be exercised in patients with certain depressive or psychiatric disorders. FDA has recently reclassified bupropion as a pregnancy category C drug, meaning that either (1) animal studies have demonstrated that the drug exerts animal-teratogenic or embryocidal effects, but there are no controlled studies in women, or (2) no studies are available in either animals or women. The pregnancy category reclassification resulted after the reanalysis of preclinical animal data demonstrated an increased incidence of fetal malformations and skeletal variations in rabbits receiving dosages approximately twofold higher than the maximum recommended human dose (on a milligram per square meter basis) of bupropion. The manufacturer continues to recommend that bupropion be used during pregnancy only if clearly needed.58
Varenicline. A partial agonist selective for the α4β2 nicotinic acetylcholine receptor, varenicline (Chantix, Pfizer, Inc., New York, NY) was approved in May 2006 for use as an aid to smoking cessation. The drug’s efficacy in smoking cessation is believed to be the result of low-level agonist activity at the receptor site combined with the competitive inhibition of nicotine binding. The partial agonist activity induces modest receptor stimulation that attenuates the symptoms of nicotine withdrawal. In addition, by blocking the ability of nicotine to activate α4β2 nicotinic acetylcholine receptors, varenicline inhibits the surges of dopamine release that are believed to be responsible for the reinforcement and reward associated with smoking.60
Data from meta-analyses suggest that the use of varenicline significantly increases long-term smoking-abstinence rates relative to placebo and sustained-release bupropion.37,61 FDA has classified varenicline as a pregnancy category C drug. The manufacturer recommends varenicline use during pregnancy only if the potential benefit justifies the potential risk to the fetus.62
In February 2008, FDA issued a public health advisory to alert health care providers about reports of serious neuropsychiatric symptoms in patients attempting to quit smoking while using varenicline, including changes in behavior, depressed mood, agitation, and suicidal ideation and behavior. The warnings and precautions sections of the drug’s prescribing information have been updated, and FDA recommends that (1) patients tell their health care providers about any history of psychiatric illness before starting varenicline and (2) clinicians and patients monitor for changes in mood and behavior during treatment with varenicline.63
Second-Line Agents. Second-line agents that have not received an FDA indication for smoking cessation include the prescription medications clonidine and nortriptyline.37 Controlled trials suggest that these agents are effective in treating tobacco dependence (Table 2), but they have a greater incidence of adverse effects and should be reserved for patients unable to quit with medications approved for smoking cessation.37
Combination Therapy. Certain combinations of medications have been shown to be effective smoking-cessation treatments. In the previous guideline, combination therapy was recommended only for patients unable to quit after monotherapy with a first-line agent. Based on data from eight clinical trials, the 2008 clinical practice guideline recommends that clinicians consider using combinations of first-line agents for patients who are willing to quit.37 Combination NRT involves the use of a long-acting formulation (e.g., nicotine patch) along with a short-acting formulation (i.e., gum, lozenge, inhaler, or nasal spray). The long-acting formulation, which delivers nicotine at relatively constant levels, is used to prevent the onset of severe withdrawal symptoms; the short-acting formulation, which delivers nicotine at a more rapid rate, is used as needed to control the withdrawal symptoms that may occur during potential relapse situations (e.g., after meals, during times of stress, when around other smokers).
Controlled trials suggest that the nicotine patch in combination with short-acting NRT formulations (i.e., gum, nasal spray, or inhaler) significantly increases quit rates relative to placebo.37 Similar results have been observed in trials using combination therapy with sustained-release bupropion and the nicotine patch. Results from an open-label trial suggest that a more-aggressive approach consisting of triple agent NRT (e.g., inhaler, patch, and nasal spray) with or without sustained-release bupropion is safe and effective among highly dependent smokers.64 Clinicians should be aware that while the combination of the nicotine patch and sustained-release bupropion has been approved by FDA, the concurrent use of two NRT products is not FDA-approved for tobacco cessation. Furthermore, the optimal combinations, dosages, and duration of dual NRTs are currently unknown. The safety and effectiveness of varenicline used with sustained-release bupropion or NRT have not been established. Pilot data suggest that patients receiving both varenicline and the nicotine patch are more likely to experience adverse effects (nausea, headache, vomiting, dizziness, dyspepsia, and fatigue) than are those receiving the patch alone.62
Smoke-Free Environments
Smoking initiation and cessation result from the interplay of numerous factors, including but not limited to environmental influences.21 Smokers in one’s environment can both promote initiation and inhibit cessation.
Data on state-specific trends in smoke-free working environments indicate that 73.4% of employees who work indoors were covered by a smoke-free workplace policy, according to a survey of 14 states conducted in 2005.65 Smoke-free workplaces have been shown to not only protect individuals from secondhand smoke but also to reduce the prevalence of smoking.66 In a simulation study, smoke-free workplace policies were estimated to be approximately nine times more cost-effective per patient than are programs that provide NRT at no cost.67
Over the past few years, numerous cities and states have adopted clean indoor air laws in workplaces.68 Nationally, the effects of transitioning all workplaces to smoke-free environments would yield an estimated 4.5% decrease in the overall prevalence of smoking.66 In its first year of implementation, a nationwide smoke-free workplace policy would produce an estimated 1.3 million new quitters, 1500 fewer myocardial infarctions, 350 fewer strokes, and a direct saving of nearly $60 million in medical costs.69 Given this substantial impact on public health, ASHP supports the implementation of smoke-free policies for the employees and patrons of all workplaces and public venues, including but not limited to offices, restaurants, bars, bowling alleys, and casinos, and for establishments where health care services are rendered. ASHP also strongly supports smoke-free campuses for all health care institutions and facilities. In practice, clinicians should educate patients about the dangers of secondhand smoke and encourage patients who continue to smoke to do so outdoors.
The Clinician’s Role
To ensure that all future clinicians have received adequate training for treating tobacco use and dependence, schools offering health-profession courses or degrees are advised to incorporate comprehensive tobacco-cessation training as part of the required curriculum for all students.37 Licensed clinicians who have not received the formal training necessary to provide comprehensive tobacco-cessation counseling are encouraged to complete continuing-education programs. Furthermore, clinicians should systematically integrate the identification of tobacco users and the delivery of evidence-based tobacco-use cessation interventions into routine patient care. In the absence of time or expertise to provide comprehensive cessation counseling, clinicians should—at a minimum—ask patients about tobacco use, advise patients to quit, and refer these patients to external resources such as the toll-free quit line (1–800-QUIT-NOW) or a group program.37 Because higher quit rates result when patients receive assistance from multiple health care providers,37 clinicians are encouraged to work in tandem with other providers as part of a team approach to helping patients quit smoking. Table 4 provides useful online links to assist clinicians in tobacco-control initiatives.
Tobacco-Cessation Resources | |
Resource | Contact Information |
Governmental agencies | |
Centers for Disease Control and Prevention | |
Environmental Protection Agency | |
Federal Trade Commission | |
Office of the Surgeon General | |
National Institutes of Health | |
National Cancer Institute | |
National Heart, Lung, and Blood Institute | |
National Institute on Drug Abuse | |
Organizations | |
American Cancer Society | |
American Heart Association | |
American Legacy Foundation | |
American Lung Association | |
American Society of Health-System Pharmacists | |
Campaign for Tobacco-Free Kids | |
Global Network of Pharmacists Against Tobacco | |
North American Quitline Consortium | |
Society for Research on Nicotine & Tobacco | |
University of California Smoking Cessation Leadership Center | |
University of California Tobacco-Related Disease Research Program | |
World Health Organization | |
Documents | |
Clinical Practice Guideline for Treating Tobacco Use and Dependence | |
Legacy Tobacco Industry Documents Archive | |
Pharmacologic aids for cessation–online support | |
Committed Quitters Program | |
Nicorette gum | |
Nicoderm patch | |
Commit lozenge | |
GETQUIT Support Plan | |
Chantix Helping Hand Program | |
Nicotrol nasal spray and inhaler | |
Smoke-Free Program | |
Generic patch (formerly Habitrol) | |
Nonpharmaceutical cessation support and programs | |
QuitKey (handheld computer for scheduled gradual reduction of smoking) | |
QuitNet (comprehensive online tobacco-cessation support program) | |
Rx for Change: Clinician-Assisted Tobacco Cessation (evidence-based tobacco-cessation training materials for educating health professional students and licensed clinicians) | |
Tobacco-Free Nurses (national program focused on helping nurses and student nurses to stop smoking) | |
Toll-free quit lines (telephone counseling for cessation, available at no cost to all callers) | 1–800-QUIT-NOW |
Given that the sale of tobacco contradicts both the clinician’s role in promoting health and the pharmacist’s code of ethics,70 ASHP strongly opposes the sale or distribution of tobacco products in all establishments where health care services are rendered (e.g., hospitals, clinics, retail chain and community pharmacies).71 For more than three decades, the pharmacy profession has repeatedly voiced opposition to the sale of tobacco products in pharmacies,72 including formal resolutions from state and national organizations. Notably, few licensed pharmacists (estimated at <2%)73 and pharmacy students (3.5%)74 are in favor of tobacco sales in pharmacies. Given this overwhelming lack of support, members of the profession are advised to insist that tobacco sales no longer occur in the environments where pharmaceutical care is rendered. Furthermore, in accordance with a 2003 resolution set forth by the American Association of Colleges of Pharmacy, ASHP encourages colleges and schools of pharmacy to give preference as clerkship sites to those pharmacies that choose not to sell tobacco products.74
Given the extensive body of data implicating tobacco as the primary known preventable cause of death in the United States, ASHP strongly supports evidence-based tobacco-control initiatives that aim to reduce the prevalence of tobacco use. These efforts should address a continuum of services, activities, and policies ranging from the integration of tobacco-cessation counseling as a routine component of patient care to the adoption of clean indoor air laws. ASHP believes that the active involvement of health care providers is crucial to effective tobacco control at the population level and that the pharmacy profession—as a primary and accessible point of contact between the health care system and the public (including the medically underserved)—has a unique opportunity to serve as a cornerstone for the nation’s tobacco-control efforts.
References
1. The health consequences of smoking: cancer. A report of the Surgeon General. http://profiles.nlm.nih.gov/NN/B/C/D/W/_/nnbcdw.pdf (accessed 2008 Jul 16).
2. Centers for Disease Control and Prevention. Annual smoking-attributable mortality, years of potential life lost, and productivity losses—United States, 1997–2001. MMWR. 2005; 54:625–8.
3. U.S. Department of Health and Human Services. 2006 Surgeon General’s report: the health consequences of involuntary exposure to tobacco smoke. www.cdc.gov/tobacco/data_statistics/sgr/sgr_2006/index.htm (accessed 2008 Jul 16).
4. Doll R, Peto R, Boreham J, et al. Mortality in relation to smoking: 50 years’ observations on male British doctors. BMJ. 2004; 328:1519.
5. Centers for Disease Control and Prevention. Cigarette smoking among adults—United States, 2000. MMWR. 2002; 51:642–5.
6. Centers for Disease Control and Prevention. Cigarette smoking among adults—United States, 2006. MMWR. 2007; 56:1157–61.
7. Lasser K, Boyd JW, Woolhandler S, et al. Smoking and mental illness: a population-based prevalence study. JAMA. 2000; 284:2606–10.
8. Centers for Disease Control and Prevention. State-specific prevalence of cigarette smoking among adults and quitting among persons aged 18–35 years—United States, 2006. MMWR. 2007; 56:993–6.
9. U.S. Department of Health and Human Services. 1994 Surgeon General’s report—preventing tobacco use among young people. www.cdc.gov/tobacco/data_statistics/sgr/sgr_1994/index.htm (accessed 2008 Aug 13).
10. U.S. Department of Health and Human Services. Healthy People 2010. Vol. I and II. www.healthypeople.gov/publications/ (accessed 2008 Jul 16).
11. Johnston LD, O’Malley PM, Bachman JG, et al. Teen smoking resumes decline. www.monitoringthefuture.org/data/07data.html#2007data-cigs (accessed 2008 May 21).
12. U.S. Department of Agriculture. Tobacco outlook. Report TBS-263. http://usda.mannlib.cornell.edu/usda/ers/TBS//2000s/2007/TBS-10–24-2007.pdf (accessed 2008 Aug 13).
13. Substance Abuse and Mental Health Services Administration Offices of Applied Studies (2008). Results from the 2007 National Survey on Drug Use and Health: National findings (DHHS publication no. SMA 08–4343). Rockville, MD.
14. Henningfield JE, Fant RV, Radzius A, et al. Nicotine concentration, smoke pH and whole tobacco aqueous pH of some cigar brands and types popular in the United States. Nicotine Tob Res. 1999; 1:163–8.
15. Connolly GN, Alpert HR, Wayne GF, et al. Trends in nicotine yield in smoke and its relationship with design characteristics among popular US cigarette brands, 1997–2005. Tob Control. 2007; 16:e5.
16. Ebbert JO, Carr AB, Dale LC. Smokeless tobacco: an emerging addiction. Med Clin North Am. 2004; 88:1593–605.
17. Centers for Disease Control and Prevention. Annual smoking-attributable mortality, years of potential life lost, and economic costs—United States, 1995–1999. MMWR. 2002; 51:300–3.
18. Best practices for comprehensive tobacco control programs—2007. Atlanta: Centers for Disease Control and Prevention; 2007.
19. Task Force on Community Preventive Services. The guide to community preventive services: tobacco use prevention and control. Am J Prev Med. 2001; 20(suppl 2):1–88.
20. U.S. Department of Health and Human Services. The health consequences of smoking: nicotine addiction: a report of the Surgeon General. http://profiles.nlm.nih.gov/NN/B/B/Z/D/_/nnbbzd.pdf (accessed 2008 Jul 16).
21. Benowitz NL. Clinical pharmacology of nicotine: implications for understanding, preventing and treating tobacco addiction. Clin Pharm Ther. 2008; 83:531–41.
22. Hughes JR, Gust SW, Skoog K, et al. Symptoms of tobacco withdrawal: a replication and extension. Arch Gen Psychiatry. 1991; 48:52–9.
23. Hughes JR. Effects of abstinence from tobacco: valid symptoms and time course. Nicotine Tob Res. 2007; 9:315–327.
24. American Psychiatric Association. Diagnostic and statistical manual of mental disorders, 4th edition. Washington, DC: American Psychiatric Publishing; 1994.
25. Benowitz NL. Cigarette smoking and nicotine addiction. Med Clin North Am. 1992; 76:415–37.
26. Hatsukami DK, Gust SW, Keenan RM. Physiologic and subjective changes from smokeless tobacco withdrawal. Clin Pharmacol Ther. 1987; 41:103–7.
27. Centers for Disease Control and Prevention. U.S. 2004 Surgeon General’s report—the health consequences of smoking. www.cdc.gov/tobacco/data_statistics/sgr/sgr_2004/index.htm (accessed 2008 Jul 16).
28. Henley SJ, Thun MJ, Connell C, et al. Two large prospective studies of mortality among men who use snuff or chewing tobacco (United States). Cancer Causes Control. 2005; 16:347–58.
29. Hergens MP, Ahlbom A, Andersson T, et al. Swedish moist snuff and myocardial infarction among men. Epidemiology. 2005; 16:12–16.
30. Gupta R, Gurm H, Bartholomew JR. Smokeless tobacco and cardiovascular risk. Arch Intern Med. 2004; 164:1845–9.
31. Henley SJ, Connell CJ, Richter P, et al. Tobacco-related disease mortality among men who switched from cigarettes to spit tobacco. Tob Control. 2007; 16:22–8.
32. California Environmental Protection Agency. Proposed identification of environmental tobacco smoke as a toxic air contaminant: executive summary. www.arb.ca.gov/regact/ets2006/ets2006.htm (accessed 2008 Oct 1).
33. Kroon LA. Drug interactions with smoking. Am J Health-Syst Pharm. 2007; 64:1917–21.
34. Zevin S, Benowitz NL. Drug interactions with tobacco smoking. Clin Pharmacokinet. 1999; 36:425–38.
35. U.S. Department of Health and Human Services. The benefits of smoking cessation. A report of the surgeon general (DHHS publication no. CDC 90–8416). U.S. Department of Health and Human Services, Public Health Service, Centers for Disease Control and Prevention and Health Promotion, Office on Smoking and Health. 1990.
36. Hazelden Foundation. Survey on current and former smokers—1998. www.hazelden.org/web/public/smokers1998.page (accessed 2008 Aug 13.).
37. Fiore MC, Jaen CR, Baker TB, et al. Treating tobacco use and dependence—2008 update. Clinical practice guideline. www.surgeongeneral.gov/tobacco/treating_tobacco_use08.pdf (accessed 2008 Jul 16).
38. Stead L, Perera R, Lancaster T. Telephone counselling for smoking cessation. Cochrane Database Syst Rev. 2006; 3:CD002850.
39. Ossip-Klein DJ, McIntosh S. Quitlines in North America: evidence base and applications. Am J Med Sci. 2003; 326:201–5.
40. Zhu SH, Anderson CM, Tedeschi GJ, et al. Evidence of real-world effectiveness of a telephone quitline for smokers. N Engl J Med. 2002; 347:1087–93.
41. Lichtenstein E, Glasgow RE, Lando HA, et al. Telephone counseling for smoking cessation: rationales and meta-analytic review of evidence. Health Educ Res. 1996; 11:243–57.
42. Hopkins DP, Briss PA, Ricard CJ, et al. Reviews of evidence regarding interventions to reduce tobacco use and exposure to environmental tobacco smoke. Am J Prev Med. 2001; 20(2, suppl):16–66.
43. Rx for Change: clinician-assisted tobacco cessation. http://rxforchange.ucsf.edu (accessed 2008 Oct 1).
44. Benowitz NL. Cigarette smoking and cardiovascular disease: pathophysiology and implications for treatment. Prog Cardiovasc Dis. 2003; 46:91–111.
45. Working Group for the Study of Transdermal Nicotine in Patients with Coronary Artery Disease. Nicotine replacement therapy for patients with coronary artery disease. Arch Intern Med. 1994; 154:989–95.
46. Joseph AM, Norman SM, Ferry LH, et al. The safety of transdermal nicotine as an aid to smoking cessation in patients with cardiac disease. N Engl J Med. 1996; 335:1792–8.
47. Tzivoni D, Keren A, Meyler S, et al. Cardiovascular safety of transdermal nicotine patches in patients with coronary artery disease who try to quit smoking. Cardiovasc Drugs Ther. 1998; 12:239–44.
48. Hubbard R, Lewis S, Smith C, et al. Use of nicotine replacement therapy and the risk of acute myocardial infarction, stroke, and death. Tob Control. 2005; 14:416–21.
49. McRobbie H, Hajek P. Nicotine replacement therapy in patients with cardiovascular disease: guidelines for health professionals. Addiction. 2001; 96:1547–51.
50. Joseph AM, Fu SS. Safety issues in pharmacotherapy for smoking in patients with cardiovascular disease. Prog Cardiovasc Dis. 2003; 45:429–41.
51. Benowitz NL, Gourlay SG. Cardiovascular toxicity of nicotine: implications for nicotine replacement therapy. J Am Coll Cardiol. 1997; 29:1422–31.
52. Meine TJ, Patel MR, Washam JB, et al. Safety and effectiveness of transdermal nicotine patch in smokers admitted with acute coronary syndromes. Am J Cardiol. 2005; 95:976–8.
53. Benowitz NL, Dempsey DA. Pharmacotherapy for smoking cessation during pregnancy. Nicotine Tob Res. 2004;6(Suppl 2):S189–202.
54. Ascher JA, Cole JO, Colin J, et al. Bupropion: a review of its mechanism of antidepressant activity. J Clin Psychiatry. 1995; 56:395–401.
55. Slemmer JE, Martin BR, Damaj MI. Bupropion is a nicotinic antagonist. J Pharmacol Exp Ther. Oct 2000; 295:321–7.
56. Hayford KE, Patten CA, Rummans TA, et al. Efficacy of bupropion for smoking cessation in smokers with a former history of major depression or alcoholism. Br J Psychiatry. 1999; 174:173–8.
57. Hughes J, Stead L, Lancaster T. Antidepressants for smoking cessation. Cochrane Database Syst Rev. 2007(1):CD000031.
58. Zyban (bupropion hydrochloride) package insert. Research Triangle Park, NC: GlaxoSmithKline, Inc.; 2007 Aug.
59. Dunner DL, Zisook S, Billow AA, et al. A prospective safety surveillance study for bupropion sustained-release in the treatment of depression. J Clin Psy-chiatry. 1998; 59:366–73.
60. Foulds J. The neurobiological basis for partial agonist treatment of nicotine dependence: varenicline. Int J Clin Pract. May 2006; 60:571–6.
61. Cahill K, Stead LF, Lancaster T. Nicotine receptor partial agonists for smoking cessation. Cochrane Database Syst Rev. 2008(3):CD006103.
62. Chantix (varenicline) package insert. New York: Pfizer, Inc.; 2008 May.
63. Food and Drug Administration. Varenicline information. www.fda.gov/cder/drug/infopage/varenicline/default.htm (accessed 2008 May 22).
64. Bars MP, Banauch GI, Appel D, et al. Tobacco Free With FDNY: the New York City Fire Department World Trade Center Tobacco Cessation Study. Chest. 2006; 129:979–87.
65. State-specific prevalence of current cigarette smoking among adults and second hand smoke rules and policies in homes and workplaces, United States, 2005. MMWR. 2006; 55:1148–51.
66. Fichtenberg CM, Glantz SA. Effect of smoke-free workplaces on smoking behaviour: systematic review. BMJ. 2002; 325:188.
67. Ong MK, Glantz SA. Free nicotine replacement therapy programs vs implementing smoke-free workplaces: a cost-effectiveness comparison. Am J Public Health. 2005; 95:969–75.
68. American Nonsmokers’ Rights Foundation. States, commonwealths, and municipalities with 100% smokefree laws in workplaces, restaurants or bars. www.no-smoke.org/pdf/100ordlist.pdf (accessed 2008 Aug 13).
69. Ong MK, Glantz SA. Cardiovascular health and economic effects of smoke-free workplaces. Am J Med. 2004; 117:32–8.
70. American Pharmacists Association. Code of ethics for pharmacists. http://www.pharmacist.com/AM/Template.cfm?Section=Code_of_Ethics_for_Pharmacists&Template=/CM/HTMLDisplay.cfm&ContentID=5420 (accessed 2008 Aug 13).
71. American Society of Health-System Pharmacists. ASHP policy position 0713: tobacco and tobacco products. http://www.ashp.org/s_ashp/docs/files/BP07/HR_Positions.pdf (accessed 2008 Aug 13).
72. Davis RM. Tobacco sales in pharmacies: mixing good drugs and bad drugs. Tob Control. 1992; 1:84–96.
73. Hudmon KS, Prokhorov AV, Corelli RL. Tobacco cessation counseling: pharmacists’ opinions and practices. Patient Educ Couns. 2006; 61:152–60.
74. Hudmon KS, Corelli RL, Fenlon CM, et al. Pharmacy student perceptions of tobacco sales in pharmacies and suggested methods for promoting tobacco-free experiential sites. Am J Pharm Educ. 2006; 70:75.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree