CHAPTER 55 Mediastinum
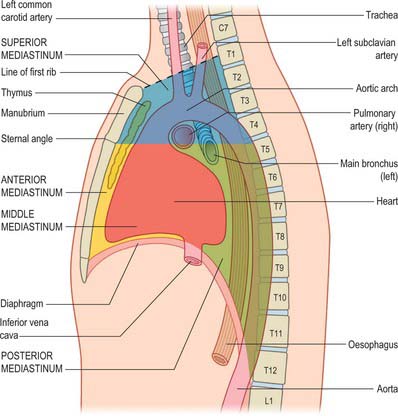
Strictly speaking, the mediastinum is the partition between the lungs and includes the mediastinal pleura. However, the term is commonly applied to the region between the two pleural sacs bounded anteriorly by the sternum and posteriorly by the thoracic vertebral column and extending vertically from the thoracic inlet to the diaphragm. For descriptive purposes, this region is arbitrarily divided into superior and inferior mediastina, and the latter is subdivided into anterior, middle and posterior parts. The plane of division into superior and inferior mediastina crosses the manubriosternal joint and the lower surface of the fourth thoracic vertebra (Fig. 55.1). Detailed accounts of some mediastinal contents are also included with descriptions of the respiratory organs (see Ch. 57) and the heart (see Ch. 56).
SUBDIVISIONS OF THE MEDIASTINUM
SUPERIOR MEDIASTINUM
The superior mediastinum lies between the manubrium sterni and the upper four thoracic vertebrae (see Fig. 55.19A–C). It is bounded below by the sternal plane, above by the plane of the thoracic inlet, and laterally by the mediastinal pleurae. It contains the lower ends of sternohyoid, sternothyroid and longus colli; thymic remnants; the internal thoracic arteries and veins, the brachiocephalic veins and the upper half of the superior vena cava, the aortic arch, the brachiocephalic, left common carotid and subclavian arteries and the left superior intercostal vein; the right and left vagi and phrenic nerves, the left recurrent laryngeal nerve, the cardiac nerves and the superficial part of the cardiac plexus; the trachea, oesophagus and thoracic duct. It also contains the paratracheal, brachiocephalic and tracheobronchial lymph nodes associated with their named structures.
INFERIOR MEDIASTINUM
Anterior mediastinum
The anterior mediastinum lies between the sternal body and pericardium (see Fig. 55.19D,E). It narrows above the fourth costal cartilages, where the pleural sacs come close to each other, and contains loose connective tissue, the sternopericardial ligaments, a few lymph nodes and the mediastinal branches of the internal thoracic artery. The anterior mediastinum may sometimes contain part of the thymus gland or its degenerated remains.
Middle mediastinum
The middle mediastinum is the broadest part of the inferior mediastinum (see Fig. 55.19D,E). It contains the pericardium, the heart and the ascending aorta; the lower half of the superior vena cava receiving the azygos venous arch posteriorly; the tracheal bifurcation and both main bronchi; the pulmonary trunk and right and left pulmonary arteries and veins; the right and left phrenic nerves; the deep part of the cardiac plexus; the tracheobronchial lymph nodes.
Posterior mediastinum
The posterior mediastinum is bounded anteriorly by the bifurcation of the trachea, pulmonary vessels, pericardium and the posterior part of the upper surface of the diaphragm (see Fig. 55.19D,E). Posteriorly, it is bounded by the vertebral column, from the lower border of the fourth to the twelfth thoracic vertebrae, and on each side by the mediastinal pleura. It contains the descending thoracic aorta and the azygos, hemiazygos and accessory azygos veins; the right and left sympathetic chains, the splanchnic nerves and the right and left vagi; the oesophagus; the thoracic duct and posterior mediastinal lymph nodes.
MEDIASTINAL COMMUNICATIONS WITH THE NECK
Anatomical pathways exist between the oral cavity and the thorax via the parapharyngeal space and other fascial planes of the neck. The parapharyngeal space is more likely to be infected than any of the other potential tissue spaces in the head and neck: infection can pass from this space to the retropharyngeal and pretracheal spaces and so reach the superior mediastinum, from where it can track down into the anterior part of the inferior mediastinum (see Ch. 28).
VASCULAR SUPPLY AND LYMPHATIC DRAINAGE
Great vessels of the superior mediastinum
The aortic arch, descending thoracic aorta, pulmonary trunk and superior vena cava are described in Chapter 56.
Azygos venous system
Azygos vein
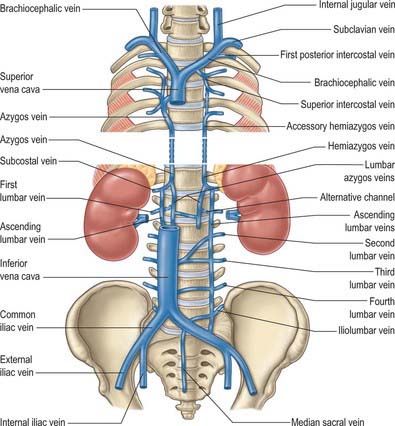
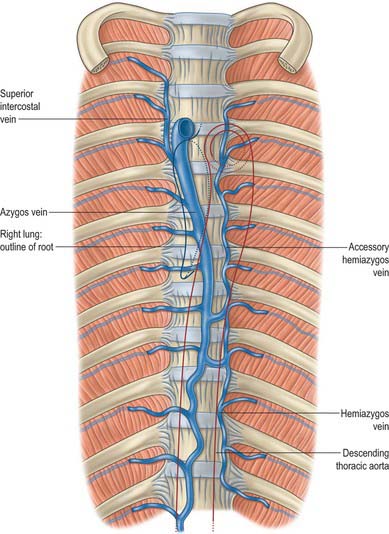
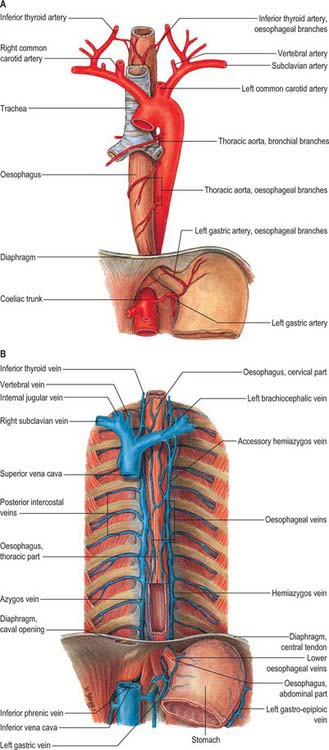
The azygos vein (Gr. azygos = ‘unpaired’) typically starts from the posterior aspect of the inferior vena cava, at or below the level of the renal veins, although the origin is not constant (Figs 55.2, 55.3; see Fig. 55.14B). When present, the lumbar azygos ascends anterior to the upper lumbar vertebrae. It may pass behind the right crus of the diaphragm or pierce it, or it may traverse the aortic hiatus to the right of the cisterna chyli. Anterior to the twelfth thoracic vertebral body, the azygos is joined by a large vessel formed by the right ascending lumbar and subcostal veins that passes forward and to the right of the twelfth thoracic vertebra behind the right crus: in the absence of a lumbar azygos, this common trunk may form the azygos vein itself. Whatever its origin, the azygos vein ascends in the posterior mediastinum to the level of the fourth thoracic vertebra, where it arches forward above the right pulmonary hilum. It ends in the superior vena cava, before the latter pierces the pericardium. The azygos lies anterior to the bodies of the lower eight thoracic vertebrae, anterior longitudinal ligament and right posterior intercostal arteries. The right greater splanchnic nerve, lung and pleura are right lateral relations. The thoracic duct and aorta and, where the vein arches forward, the oesophagus, trachea and right vagus, are left lateral relations. In the lower thorax the azygos is covered anteriorly by a recess of the right pleural sac and by the oesophagus; it emerges from behind the oesophagus to ascend behind the right hilum. The azygos vein lies close to the right posterolateral aspect of the descending thoracic aorta: aortic pulsations may assist venous return in the azygos and hemiazygos veins.
Hemiazygos vein
The hemiazygos vein is formed on the left side from the lower three posterior intercostal veins, a common trunk formed by the left ascending lumbar and subcostal veins, and by oesophageal and mediastinal tributaries (Fig. 55.2). It ascends anterior to the level of the vertebral column to the eighth thoracic level then crosses the vertebral column posterior to the aorta, oesophagus and thoracic duct, and ends in the azygos vein (Fig. 55.3). Its lower end is often connected to the left renal vein.
Accessory hemiazygos vein
The accessory hemiazygos vein descends to the left of the vertebral column, and receives veins from the fourth or fifth to eighth left intercostal spaces; it crosses the seventh thoracic vertebra to join the azygos vein (see Figs 55.5 and 55.6). The accessory hemiazygos vein sometimes receives the left bronchial veins, and it may join the hemiazygos vein, in which case their common trunk opens into the azygos vein.
Variations of the azygos veins
The azygos veins vary greatly in their mode of origin, course, tributaries, anastomoses and termination. The accessory hemiazygos is the most variable, and may drain into the left brachiocephalic, azygos or hemiazygos vein. The arrangement shown in Fig. 55.3 represents a common pattern. Commonly, there is a main ‘right-sided’ azygos and at least some representative of the hemiazygos veins. The latter vary, and one or other may be absent or poorly developed. Very occasionally, independent left and right azygos veins (the early embryonic form) persist, or a single azygos vein may occur in a midline position without hemiazygos tributaries. Retro-aortic transvertebral connections from hemiazygos and accessory hemiazygos veins to the azygos are also extremely variable: there may be up to five connections. When either of the hemiazygos veins is absent, the relevant intercostal veins cross the vertebral bodies and end in the azygos. These transvertebral routes are often very short, because the azygos vein is more commonly anterior to the vertebral column and often passes to the left of the midline for part of its course. When there is congenital interruption of the inferior vena cava (IVC), the azygos vein can become as large as the IVC that it has replaced. Rarely, the azygos arch in the right tracheobronchial angle can be placed more superolaterally in a diagonal accessory azygos fissure in the right upper lobe of the lung as a consequence of failure of embryonic descent.
MEDIASTINAL LYMPH NODES
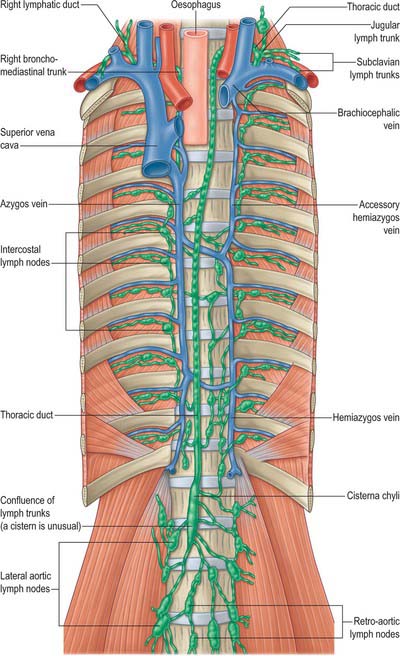
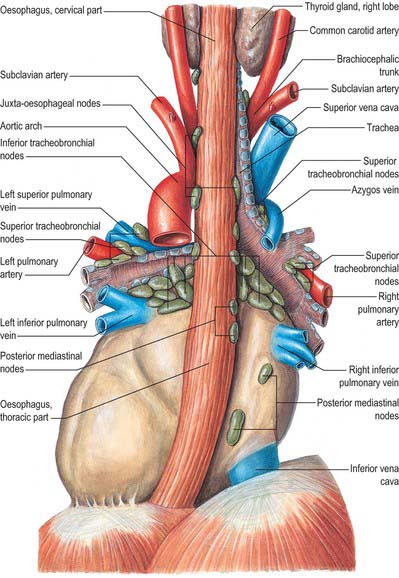
The mediastinal lymph nodes (Fig. 55.4; see Fig. 56.3) are classified into regional lymph node stations by thoracic surgeons for the purposes of staging lung cancer. The stations are defined as follows: Station 1: highest mediastinal nodes lie above a horizontal line of the level at which the left brachiocephalic vein crosses the trachea. Station 2: upper paratracheal nodes lie below the line of the highest mediastinal nodes and above a line drawn horizontally at the level of the upper border of the aortic arch. Station 3: prevascular and retrotracheal nodes lie behind the trachea but in front of the great vessels. Station 4: lower paratracheal nodes lie below the upper margin of the aortic arch and down to the upper margin of the corresponding upper lobe bronchus. On the right side, this is the upper margin of the right upper lobe bronchus; the majority of nodes in this area tend to be positioned anterolateral to the trachea. On the left side, the nodes are located below the upper margin of the aortic arch and above the margin of the left upper lobe bronchus. They lie medial to the ligamentum arteriosum and are usually lateral to the trachea. Station 5: subaortic nodes lie in the aortopulmonary window and are situated lateral to the ligamentum arteriosum or aorta or left pulmonary artery, but proximal to the first division of the left pulmonary artery. Station 6: para-aortic nodes lie between the upper margin of the aortic arch and lateral to the ascending aorta and aortic arch. Station 7: subcarinal nodes lie below the carina of the trachea, but are not associated with the lower lobe bronchi. Station 8: para-oesophageal nodes lie at either side of the oesophagus, well below the level of the subcarinal nodes. Station 9: pulmonary ligament nodes lie within the pulmonary ligament.
Involvement of these lymph nodes by cancer cells has important prognostic implications and influences the choice of treatment (Mountain & Dresler 1997). The staging system for lung cancer classifies involvement of ipsilateral hilar lymph nodes as N1, ipsilateral mediastinal lymph nodes as N2, contralateral mediastinal/hilar nodes as N3 and supraclavicular/scalene nodes as M1 (distant metastases).
These groups are not sharply demarcated. Pulmonary nodes become continuous with the bronchopulmonary nodes, and these in turn merge with the inferior and superior tracheobronchial nodes, which are continuous with the paratracheal group. Afferents of tracheobronchial nodes drain the lungs and bronchi, the thoracic part of the trachea, the heart and some efferents of the posterior mediastinal nodes. Their efferent vessels ascend on the trachea to unite with efferents of the parasternal and brachiocephalic nodes as the right and left bronchomediastinal trunks. The right trunk may occasionally join a right lymphatic duct or another right-sided lymph trunk and the left trunk may join the thoracic duct, but usually they open independently in or near the ipsilateral jugulosubclavian junction (see Ch. 28).
THORACIC DUCT
In adults, the thoracic duct, including the confluence of lymph trunks (or the cisterna chyli in the small proportion of individuals in whom the latter is saccular), is 38–45 cm in length and extends from the second lumbar vertebra to the base of the neck (Fig. 55.5). Starting from the superior pole of the confluence near the lower border of the twelfth thoracic vertebra (see Ch. 62), the thoracic duct traverses the retrocrural space of the diaphragm with the aorta, azygos and hemiazygos veins, then ascends in the posterior mediastinum, on the right of the midline, between the descending thoracic aorta (on its left) and the azygos vein (on its right). The vertebral column, the right aortic intercostal arteries and terminal segments of the hemiazygos and accessory hemiazygos veins are posterior relations. The diaphragm and oesophagus are anterior; a recess of the right pleural cavity may separate the duct and oesophagus. At the level of the body of the fifth thoracic vertebra, the duct gradually inclines to the left, enters the superior mediastinum and then ascends to the thoracic inlet along the left border of the oesophagus. In this part of its course the duct is first crossed anteriorly by the aortic arch and then runs posterior to the initial segment of the left subclavian artery, in close contact with the left mediastinal pleura. Passing into the neck, it arches laterally at the level of the transverse process of the seventh cervical vertebra. Its arch rises 3 or 4 cm above the clavicle and curves anterior to the vertebral artery and vein, the left sympathetic trunk, the thyrocervical trunk or its branches, the left phrenic nerve and the medial border of scalenus anterior. It passes posterior to the left common carotid artery, vagus nerve and internal jugular vein. Finally, the duct descends anterior to the arched cervical first part of the left subclavian artery and ends by opening into the junction of the left subclavian and internal jugular veins (see Fig. 28.16A). It may also open into either of the great veins, near the junction, or it may divide into a number of smaller vessels before terminating. In rare individuals there is no apparent thoracic duct on the left. Several terminal openings sometimes occur. Patterns vary greatly in different studies, but the commonly reported sites of termination are the internal jugular vein, jugulosubclavian junction and subclavian vein. Termination in the left brachiocephalic vein occurs occasionally.
Anomalies of the thoracic duct may be delineated by dissection into the inguinal lymphatics followed by cannulation and subsequent injection of an oily contrast medium (lipiodol) followed by plain films or CT.
INJURY DURING OESOPHAGEAL SURGERY
The thoracic duct is vulnerable to damage after thoracic, particularly oesophageal, surgery (Wemyss-Holden et al 2001). The incidence is between 0.2% and 3% and increases with transhiatal and thoracoscopic procedures. Thoracic duct laceration is a potentially life-threatening complication: mortality rates are more than 50% with conservative management and as high as 10–16% even after early surgical duct ligation. Rupture of the thoracic duct leads to leakage of chyle, which is rich in lipid, protein and lymphocytes, and hence a progressive nutritional and immune deficit occurs. Fortunately, the incidence of true thoracic duct transection is rare, and cases in which there are some postoperative chylous effusions are usually the result of damage to some of the tributaries of the thoracic duct, rather than to the duct itself. These are usually self-limiting and respond to conservative treatment.
RIGHT LYMPHATIC TRUNK
The right lymphatic trunk (Fig. 55.5) has a variable anatomy, which includes doubling of the duct, left-sided, right or bilateral termination. The plexiform nature of the trunks from which the thoracic duct develops leads to a number of possible abnormalities. The most common is duplication of the duct for a variable part of its course and subsequent merging to form a single duct which drains into the left internal jugular trunk. Occasionally the duct has dual terminations in the right and left internal jugular veins, and rarely it terminates only into the right internal jugular vein.
AUTONOMIC NERVOUS SYSTEM
The autonomic nervous system in the thorax consists of right and left ganglionated sympathetic chains and vagi and the cardiac, pulmonary and oesophageal plexuses. The cardiac plexus is described in Chapter 56, the pulmonary plexus is described in Chapter 57, and the oesophageal plexus is described below.
THORACIC SYMPATHETIC TRUNK
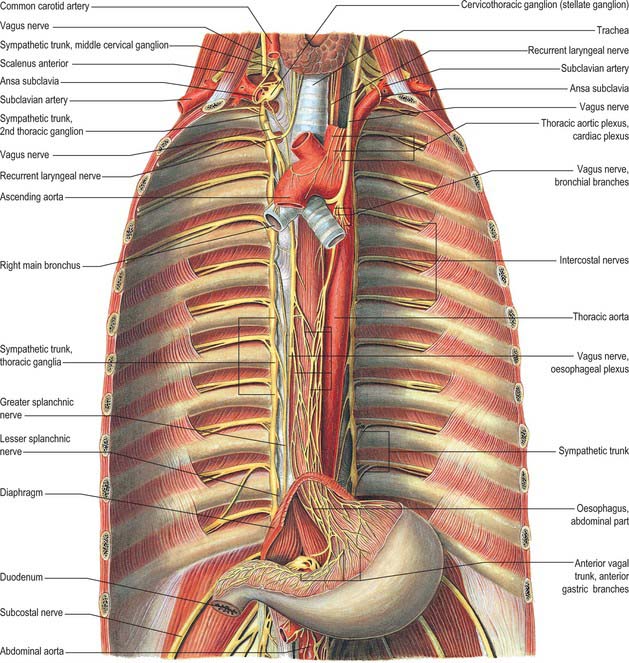
The thoracic sympathetic trunk (Fig. 55.7; see Fig. 15.13) contains ganglia almost equal in number to those of the thoracic spinal nerves (usually 11, occasionally 12, rarely 10 or 13). Almost always, the first thoracic ganglion is fused with the inferior cervical ganglion, forming the cervicothoracic ganglion. Occasionally, the second thoracic ganglion may be included in this fusion, in which case the succeeding ganglion is counted as the second in order to make the other ganglia correspond numerically with other segmental structures. Except for the second and lowest two or three, the thoracic ganglia lie against the costal heads, posterior to the costal pleura. The second thoracic sympathetic ganglion is commonly located in the second intercostal space, and the lowest two or three ganglia lie lateral to the bodies of the corresponding vertebrae. Caudally, the thoracic sympathetic trunk passes dorsal to the medial arcuate ligament (or through the crus of the diaphragm) to become the lumbar sympathetic trunk.
The ganglia are small and interconnected by intervening segments of the trunk. The classic description is that two or more rami communicantes, named white and grey, connect each ganglion with its corresponding spinal nerve, the white rami joining the nerve distal to the grey. Variation in this pattern is not uncommon, especially in the upper thoracic levels: these variations are rarely bilaterally symmetrical. Additional ascending and descending rami to higher and lower levels respectively issue from the second sympathetic ganglion (54% and 46% respectively), from the third ganglion (6% and 25%), and from the fourth ganglion (5% and 8%) (Cho et al 2005). The ascending ramus from the second ganglion is sometimes called the nerve of Kuntz. The anatomical variations displayed by the communicating rami and in the location of the second sympathetic ganglion might explain some surgical failures and symptom recurrences). Occasionally, a grey and a white ramus fuse to form a ‘mixed’ ramus.
The medial branches from the upper five ganglia are very small, and supply filaments to the thoracic aorta and its branches; they form a fine thoracic aortic plexus on the aorta with filaments from the greater splanchnic nerve. Rami of the second to fifth or sixth ganglia enter the posterior pulmonary plexus. Others, from the second to fifth ganglia, pass to the deep (dorsal) part of the cardiac plexus. Small branches of these pulmonary and cardiac nerves pass to the oesophagus and trachea. The medial branches from the lower seven ganglia are large; they supply the aorta and unite to form the greater, lesser and lowest splanchnic nerves (the last two are not always identifiable).
Thoracic sympathectomy
The effect is immediately evident: the patient awakes from anaesthesia with dry and warm hands. In many cases, even hyperhidrosis of the feet improves, but the underlying anatomical/physiological mechanisms are not yet properly understood (Gofeld & Faclier 2006). Surgical complications are very rare: Horner’s syndrome is the most feared, and is caused by damage to the stellate ganglion and interruption of the sympathetic fibres from T1 which ascend around the arteries supplying the head and neck (see Ch. 28).