15
Gram-Positive Cocci
CHAPTER CONTENTS
INTRODUCTION
There are two medically important genera of gram-positive cocci: Staphylococcus and Streptococcus. Two of the most important human pathogens, Staphylococcus aureus and Streptococcus pyogenes, are described in this chapter. Staphylococci and streptococci are nonmotile and do not form spores.
Both staphylococci and streptococci are gram-positive cocci, but they are distinguished by two main criteria:
(1) Microscopically, staphylococci appear in grapelike clusters, whereas streptococci are in chains.
(2) Biochemically, staphylococci produce catalase (i.e., they degrade hydrogen peroxide), whereas streptococci do not.
STAPHYLOCOCCUS
Diseases
Staphylococcus aureus causes abscesses (Figure 15–1), various pyogenic infections (e.g., endocarditis, septic arthritis, and osteomyelitis), food poisoning, scalded skin syndrome (Figure 15–2), and toxic shock syndrome. It is one of the most common causes of hospital-acquired pneumonia, septicemia, and surgical-wound infections. It is an important cause of skin infections, such as folliculitis (Figure 15–3), cellulitis, and impetigo (Figure 15–4). It is the most common cause of bacterial conjunctivitis.
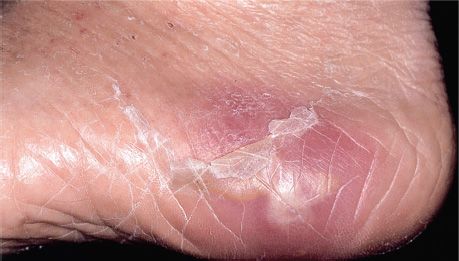
FIGURE 15–1 Abscess on foot. Note central raised area of whitish pus surrounded by erythema. An abscess is the classic lesion caused by Staphylococcus aureus. (Used with permission from Wolff K, Johnson R (eds): Fitzpatrick’s Color Atlas & Synopsis of Clinical Dermatology. 6th ed. New York: McGraw-Hill, 2009. Copyright © 2009 by The McGraw-Hill Companies, Inc.)
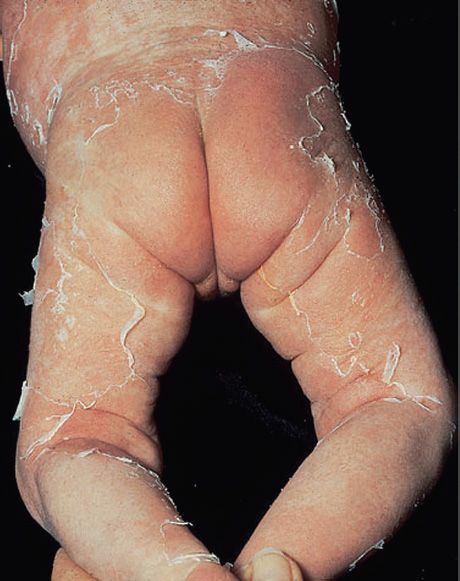
FIGURE 15–2 Scalded skin syndrome. Note widespread areas of “rolled up” desquamated skin in infant. Caused by an exotoxin produced by Staphylococcus aureus. (Used with permission from Wolff K, Johnson R (eds): Fitzpatrick’s Color Atlas & Synopsis of Clinical Dermatology. 6th ed. New York: McGraw-Hill, 2009. Copyright © 2009 by The McGraw-Hill Companies, Inc.)
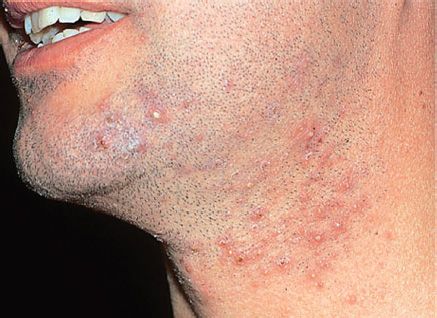
FIGURE 15–3 Folliculitis. Note the multiple, small pustules on the chin and neck. Staphylococcus aureus is the most common cause of folliculitis. (Reproduced with permission from Wolff K, Goldsmith LA, Katz SI et al (eds): Fitzpatrick’s Dermatology in General Medicine. 7th ed. New York: McGraw-Hill, 2008, pg 1699. Copyright © 2008 by The McGraw-Hill Companies, Inc.)
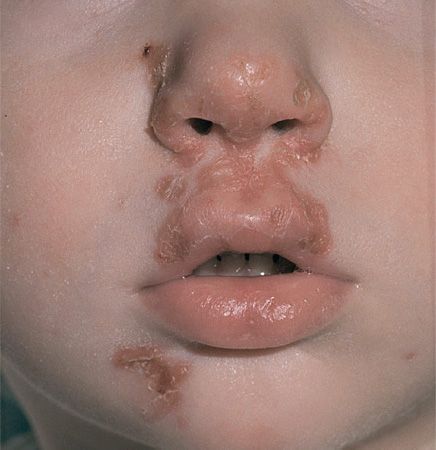
FIGURE 15–4 Impetigo. Lesions of impetigo are crops of vesicles with a “honey-colored” crust. Impetigo is caused by either Staphylococcus aureus or Streptococcus pyogenes. (Used with permission from Wolff K, Johnson R (eds): Fitzpatrick’s Color Atlas & Synopsis of Clinical Dermatology. 6th ed. New York: McGraw-Hill, 2009. Copyright © 2009 by The McGraw-Hill Companies, Inc.)
Staphylococcus epidermidis can cause endocarditis and prosthetic joint infections. Staphylococcus saprophyticus causes urinary tract infections. Kawasaki syndrome is a disease of unknown etiology that may be caused by certain strains of S. aureus.
Important Properties
Staphylococci are spherical gram-positive cocci arranged in irregular grapelike clusters (Figure 15–5). All staphylococci produce catalase, whereas no streptococci do (catalase degrades H2O2 into O2 and H2O). Catalase is an important virulence factor. Bacteria that make catalase can survive the killing effect of H2O2 within neutrophils.
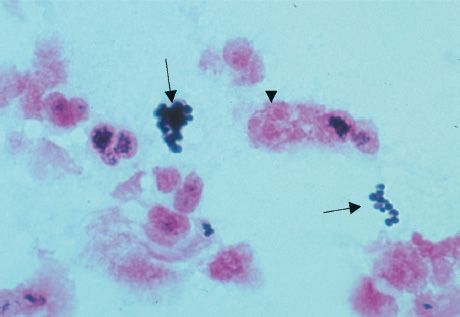
FIGURE 15–5 Staphylococcus aureus—Gram stain. Arrows point to two “grapelike” clusters of gram-positive cocci. Arrowhead points to neutrophil with pink segmented nuclei. (Used with permission from Professor Shirley Lowe, University of California, San Francisco School of Medicine.)
Three species of staphylococci are human pathogens: S. aureus, S. epidermidis, and S. saprophyticus (Table 15–1). Of the three, S. aureus is by far the most important. S. aureus is distinguished from the others primarily by coagulase production (Figure 15–6). Coagulase is an enzyme that causes plasma to clot by activating prothrombin to form thrombin. Thrombin then catalyzes the activation of fibrinogen to form the fibrin clot. S. epidermidis and S. saprophyticus are often referred to as coagulase-negative staphylococci.
TABLE 15–1 Staphylococci of Medical Importance
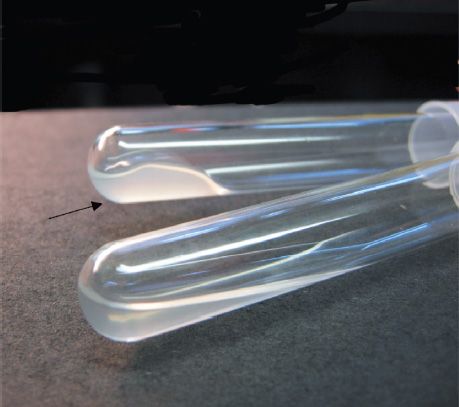
FIGURE 15–6 Coagulase test—Upper tube inoculated with Staphylococcus aureus; lower tube inoculated with Staphylococcus epidermidis. Arrow points to clotted plasma formed by coagulase produced by S. aureus. (Used with permission from Professor Shirley Lowe, University of California, San Francisco School of Medicine.)
S. aureus produces a carotenoid pigment called staphyloxanthin, which imparts a golden color to its colonies. This pigment enhances the pathogenicity of the organism by inactivating the microbicidal effect of superoxides and other reactive oxygen species within neutrophils. S. epidermidis does not synthesize this pigment and produces white colonies. The virulence of S. epidermidis is significantly less than that of S. aureus.
Two other characteristics further distinguish these species, namely, S. aureus usually ferments mannitol and hemolyzes red blood cells, whereas S. epidermidis and S. saprophyticus do not. Hemolysis of red cells by hemolysins produced by S. aureus is the source of iron required for growth of the organism. The iron in hemoglobin is recovered by the bacteria and utilized in the synthesis of cytochrome enzymes used to produce energy.
More than 90% of S. aureus strains contain plasmids that encode β-lactamase, the enzyme that degrades many, but not all, penicillins. Some strains of S. aureus are resistant to the β-lactamase–resistant penicillins, such as methicillin and nafcillin, by virtue of changes in the penicillin-binding protein (PBP) in their cell membrane. Genes on the bacterial chromosome called mecA genes encode these altered PBPs.
These strains are commonly known as methicillin-resistant S. aureus (MRSA) or nafcillin-resistant S. aureus (NRSA). MRSA currently accounts for more than 50% of S. aureus strains isolated from hospital patients in the United States. The most common strain of MRSA in the United States is the “USA300” strain.
Strains of S. aureus with intermediate resistance to vancomycin (VISA) and with full resistance to vancomycin (VRSA) have also been detected. The cassette of genes that encodes vancomycin resistance in S. aureus is the same as the cassette that provides vancomycin resistance in enterococci. These genes are located in a transposon on a plasmid and encode the enzymes that substitute D-lactate for D-alanine in the peptidoglycan.
S. aureus has several important cell wall components and antigens:
(1) Protein A is the major protein in the cell wall. It is an important virulence factor because it binds to the Fc portion of IgG at the complement-binding site, thereby preventing the activation of complement. As a consequence, no C3b is produced, and the opsonization and phagocytosis of the organisms are greatly reduced. Protein A is used in certain tests in the clinical laboratory because it binds to IgG and forms a “coagglutinate” with antigen–antibody complexes. The coagulase-negative staphylococci do not produce protein A.
(2) Teichoic acids are polymers of ribitol phosphate. They mediate adherence of the staphylococci to mucosal cells. Lipoteichoic acids play a role in the induction of septic shock by inducing cytokines such as interleukin-1 (IL-1) and tumor necrosis factor (TNF) from macrophages (See the discussion of septic shock in the Endotoxin section of Chapter 7).
(3) Polysaccharide capsule is also an important virulence factor. There are 11 serotypes based on the antigenicity of the capsular polysaccharide, but types 5 and 8 cause 85% of infections. Some strains of S. aureus are coated with a small amount of polysaccharide capsule, called a microcapsule. The capsule is poorly immunogenic, which has made producing an effective vaccine difficult.
(4) Surface receptors for specific staphylococcal bacteriophages permit the “phage typing” of strains for epidemiologic purposes. Teichoic acids make up part of these receptors.
(5) The peptidoglycan of S. aureus has endotoxin-like properties (i.e., it can stimulate macrophages to produce cytokines and can activate the complement and coagulation cascades). This explains the ability of S. aureus to cause the clinical findings of septic shock yet not possess endotoxin.
Transmission
Humans are the reservoir for staphylococci. The nose is the main site of colonization of S. aureus, and approximately 30% of people are colonized at any one time. People who are chronic carriers of S. aureus in their nose have an increased risk of skin infections caused by S. aureus.
The skin, especially of hospital personnel and patients, is also a common site of S. aureus colonization. Hand contact is an important mode of transmission, and handwashing decreases transmission.
S. aureus is also found in the vagina of approximately 5% of women, which predisposes them to toxic shock syndrome. Additional sources of staphylococcal infection are shedding from human lesions and fomites such as towels and clothing contaminated by these lesions.
Disease caused by S. aureus is favored by a heavily contaminated environment (e.g., family members with boils) and a compromised immune system. Reduced humoral immunity, including low levels of antibody, complement, or neutrophils, especially predisposes to staphylococcal infections. Diabetes and intravenous drug use predispose to infections by S. aureus. Patients with chronic granulomatous disease (CGD), a disease characterized by a defect in the ability of neutrophils to kill bacteria, are especially prone to S. aureus infections (see Chapter 68).
S. epidermidis is found primarily on the human skin and can enter the bloodstream at the site of intravenous catheters that penetrate through the skin. S. saprophyticus is found primarily on the mucosa of the genital tract in young women and from that site can ascend into the urinary bladder to cause urinary tract infections.
Pathogenesis
Staphylococcus aureus
S. aureus causes disease both by producing toxins and by inducing pyogenic inflammation. The typical lesion of S. aureus infection is an abscess. Abscesses undergo central necrosis and usually drain to the outside (e.g., furuncles and boils), but organisms may disseminate via the bloodstream as well. Foreign bodies, such as sutures and intravenous catheters, are important predisposing factors to infection by S. aureus.
Several important toxins and enzymes are produced by S. aureus. The three clinically important exotoxins are enterotoxin, toxic shock syndrome toxin, and exfoliatin.
(1) Enterotoxin causes food poisoning characterized by prominent vomiting and watery, nonbloody diarrhea. It acts as a superantigen within the gastrointestinal tract to stimulate the release of large amounts of IL-1 and IL-2 from macrophages and helper T cells, respectively. The prominent vomiting appears to be caused by cytokines released from the lymphoid cells, which stimulate the enteric nervous system to activate the vomiting center in the brain. Enterotoxin is fairly heat-resistant and is therefore usually not inactivated by brief cooking. It is resistant to stomach acid and to enzymes in the stomach and jejunum. There are six immunologic types of enterotoxin, types A–F.
(2) Toxic shock syndrome toxin (TSST) causes toxic shock, especially in tampon-using menstruating women or in individuals with wound infections. Toxic shock also occurs in patients with nasal packing used to stop bleeding from the nose. TSST is produced locally by S. aureus in the vagina, nose, or other infected site. The toxin enters the bloodstream, causing a toxemia. Blood cultures typically do not grow S. aureus.
TSST is a superantigen and causes toxic shock by stimulating the release of large amounts of IL-1, IL-2, and TNF (see the discussions of exotoxins in Chapter 7 and superantigens in Chapter 58). Approximately 5% to 25% of isolates of S. aureus carry the gene for TSST. Toxic shock occurs in people who do not have antibody against TSST.
(3) Exfoliatin causes “scalded skin” syndrome in young children. It is “epidermolytic” and acts as a protease that cleaves desmoglein in desmosomes, leading to the separation of the epidermis at the granular cell layer.
(4) Several exotoxins can kill leukocytes (leukocidins) and cause necrosis of tissues in vivo. Of these, the two most important are alpha toxin and P-V leukocidin. Alpha toxin causes marked necrosis of the skin and hemolysis. The cytotoxic effect of alpha toxin is attributed to the formation of holes in the cell membrane and the consequent loss of low-molecular-weight substances from the damaged cell.
P-V leukocidin is a pore-forming toxin that kills cells, especially white blood cells, by damaging cell membranes. The two subunits of the toxin assemble in the cell membrane to form a pore through which cell contents leak out. The gene encoding P-V leukocidin is located on a lysogenic phage. The importance of P-V leukocidin as a virulence factor is indicated by the severe skin and soft tissue infection caused by MRSA strains that produce this leukocidin. A severe necrotizing pneumonia is also caused by strains of S. aureus that produce P-V leukocidin. Approximately 2% of clinical isolates of S. aureus produce P-V leukocidin.
(5) The enzymes include coagulase, fibrinolysin, hyaluronidase, proteases, nucleases, and lipases. Coagulase, by clotting plasma, serves to wall off the infected site, thereby retarding the migration of neutrophils into the site. Staphylokinase is a fibrinolysin that can lyse thrombi.
Staphylococcus epidermidis & Staphylococcus saprophyticus
Unlike S. aureus, these two coagulase-negative staphylococci do not produce exotoxins. Thus, they do not cause food poisoning or toxic shock syndrome. They do, however, cause pyogenic infections. For example, S. epidermidis is a prominent cause of pyogenic infections on prosthetic implants such as heart valves and hip joints, and S. saprophyticus causes urinary tract infections, especially cystitis.
Clinical Findings
The important clinical manifestations caused by S. aureus can be divided into two groups: pyogenic (pus-producing) and toxin-mediated (Table 15–2). S. aureus is a major cause of skin, soft tissue, bone, joint, lung, heart, and kidney infections. Pyogenic diseases are the first group described, and toxin-mediated diseases are the second group.
TABLE 15–2 Important Features of Pathogenesis by Staphylococci
Staphylococcus aureus: Pyogenic Diseases
(1) Skin infections are very common. These include impetigo (Figure 15–4), furuncles, carbuncles (Figure 15–7), paronychia cellulitis, folliculitis (Figure 15–3), hidradenitis suppurativa, conjunctivitis, eyelid infections (blepharitis and hordeolum), and postpartum breast infections (mastitis). Lymphangitis can occur, especially on the forearm associated with an infection on the hand.
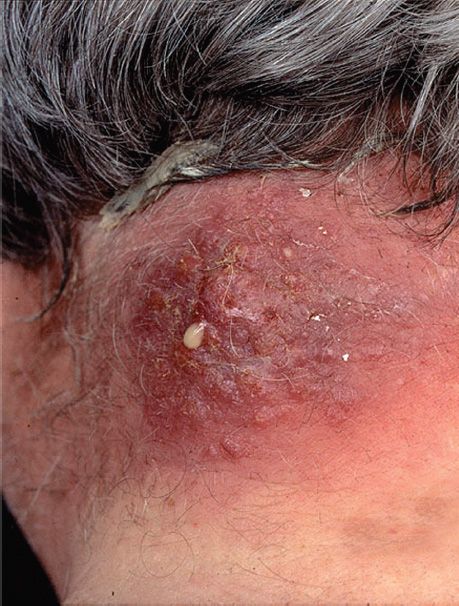
FIGURE 15–7 Carbuncle. A carbuncle is a multiheaded abscess often located on the back of the neck. Note drop of yellowish pus near the center of the lesion. Carbuncles are caused by Staphylococcus aureus. (Used with permission from Wolff K, Johnson R (eds): Fitzpatrick’s Color Atlas & Synopsis of Clinical Dermatology. 6th ed. New York: McGraw-Hill, 2009. Copyright © 2009 by The McGraw-Hill Companies, Inc.)
Severe necrotizing skin and soft tissue infections are caused by MRSA strains that produce P-V leukocidin. These infections are typically community-acquired rather than hospital-acquired. These community-acquired, methicillin-resistant strains of S. aureus (CA-MRSA) are a common cause of infection among the homeless and intravenous drug users. Athletes who engage in close personal contact such as wrestlers and football players are also at risk. Note that hospital-acquired MRSA (HA-MRSA) causes approximately 50% of all nosocomial S. aureus infections. Molecular analysis reveals that the CA-MRSA strains are different from the HA-MRSA strains.
(2) Septicemia (sepsis) can originate from any localized lesion, especially wound infection, or as a result of intravenous drug abuse. Sepsis caused by S. aureus has clinical features similar to those of sepsis caused by certain gram-negative bacteria, such as Neisseria meningitidis (see Chapter 16).
(3) Endocarditis may occur on normal or prosthetic heart valves, especially right-sided endocarditis (tricuspid valve) in intravenous drug users. (Prosthetic valve endocarditis is often caused by S. epidermidis.)
(4) Osteomyelitis and septic arthritis may arise either by hematogenous spread from a distant infected focus or be introduced locally at a wound site. S. aureus is a very common cause of these diseases, especially in children.
(5) Postsurgical wound infections are an important cause of morbidity and mortality in hospitals. S. aureus is the most common cause. For example, S. aureus and S. epidermidis are the most common causes of infections at the site where cardiac pacemakers are installed.
(6) Pneumonia can occur in postoperative patients or following viral respiratory infection, especially influenza. Staphylococcal pneumonia often leads to empyema or lung abscess. In many hospitals, it is the most common cause of nosocomial pneumonia in general and especially of ventilator-associated pneumonia in intensive care units. CA-MRSA causes a severe necrotizing pneumonia.
(7) Conjunctivitis typically presents with unilateral burning eye pain, hyperemia of the conjunctiva, and a purulent discharge. The organism is transmitted to the eye by contaminated fingers. S. aureus is the most common cause overall, but Streptococcus pneumoniae and Haemophilus influenzae are more common in children. Gonococcal and nongonococcal (caused by Chlamydia trachomatis) conjunctivitis is acquired by infants during passage through the birth canal.
(8) Abscesses can occur in any organ when S. aureus circulates in the bloodstream (bacteremia). These abscesses are often called “metastatic abscesses” because they occur by the spread of bacteria from the original site of infection, often in the skin.
Staphylococcus aureus: Toxin-Mediated Diseases
(1) Food poisoning (gastroenteritis) is caused by ingestion of enterotoxin, which is preformed in foods and hence has a short incubation period (1–8 hours). In staphylococcal food poisoning, vomiting is typically more prominent than diarrhea.
(2) Toxic shock syndrome is characterized by fever; hypotension; a diffuse, macular, sunburn-like rash that goes on to desquamate; and involvement of three or more of the following organs: liver, kidney, gastrointestinal tract, central nervous system, muscle, or blood.
(3) Scalded-skin syndrome is characterized by fever, large bullae, and an erythematous macular rash. Large areas of skin slough, serous fluid exudes, and electrolyte imbalance can occur. Hair and nails can be lost. Recovery usually occurs within 7–10 days. This syndrome occurs most often in young children.
Staphylococcus aureus: Kawasaki Disease
Kawasaki disease (KD) is a disease of unknown etiology that is discussed here because several of its features resemble toxic shock syndrome caused by the superantigens of S. aureus (and S. pyogenes). KD is a vasculitis involving small and medium-size arteries, especially the coronary arteries. It is the most common cause of acquired heart disease in children in the United States.
Clinically, KD is characterized by a high fever of at least 5 days’ duration; bilateral nonpurulent conjunctivitis; lesions of the lips and oral mucosa (e.g., strawberry tongue, edema of the lips, and erythema of the oropharynx); cervical lymphadenopathy; a diffuse erythematous, maculopapular rash; and erythema and edema of the hands and feet that often ends with desquamation.
The most characteristic clinical finding of KD is cardiac involvement, especially myocarditis, arrhythmias, and regurgitation involving the mitral or aortic valves. The main cause of morbidity and mortality in KD is aneurysm of the coronary arteries.
KD is much more common in children of Asian ancestry, leading to speculation that certain major histocompatibility complex (MHC) alleles may predispose to the disease. It is a disease of children younger than 5 years of age, often occurring in mini-outbreaks. It occurs worldwide but is much more common in Japan.
There is no definitive diagnostic laboratory test for KD. Effective therapy consists of high-dose immune globulins (IVIG) plus high-dose aspirin, which promptly reduce the fever and other symptoms and, most importantly, significantly reduce the occurrence of aneurysms.
Staphylococcus epidermidis & Staphylococcus saprophyticus
There are two coagulase-negative staphylococci of medical importance: S. epidermidis and S. saprophyticus. S. epidermidis infections are almost always hospital-acquired, whereas S. saprophyticus infections are almost always community-acquired.
S. epidermidis is part of the normal human flora on the skin and mucous membranes but can enter the bloodstream (bacteremia) and cause metastatic infections, especially at the site of implants. It commonly infects intravenous catheters and prosthetic implants (e.g., prosthetic heart valves [endocarditis], vascular grafts, and prosthetic joints [arthritis or osteomyelitis]) (Table 15–2). S. epidermidis is also a major cause of sepsis in neonates and of peritonitis in patients with renal failure who are undergoing peritoneal dialysis through an indwelling catheter. It is the most common bacterium to cause cerebrospinal fluid shunt infections.
Strains of S. epidermidis that produce a glycocalyx are more likely to adhere to prosthetic implant materials and therefore are more likely to infect these implants than strains that do not produce a glycocalyx. Hospital personnel are a major reservoir for antibiotic-resistant strains of S. epidermidis.
S. saprophyticus causes urinary tract infections, particularly in sexually active young women. Most women with this infection have had sexual intercourse within the previous 24 hours. This organism is second to Escherichia coli as a cause of community-acquired urinary tract infections in young women.
Laboratory Diagnosis
Smears from staphylococcal lesions reveal gram-positive cocci in grapelike clusters (Figure 15–5). Cultures of S. aureus typically yield golden-yellow colonies that are usually β-hemolytic. S. aureus is coagulase-positive (Figure 15–6). Mannitol-salt agar is a commonly used screening device for S. aureus. Cultures of coagulase-negative staphylococci typically yield white colonies that are nonhemolytic. The two coagulase-negative staphylococci are distinguished by their reaction to the antibiotic novobiocin: S. epidermidis is sensitive, whereas S. saprophyticus is resistant. There are no serologic or skin tests used for the diagnosis of any acute staphylococcal infection.
In toxic shock syndrome, isolation of S. aureus is not required to make a diagnosis as long as the clinical criteria are met. Laboratory findings that support a diagnosis of toxic shock syndrome include the isolation of a TSST-producing strain of S. aureus and development of antibodies to the toxin during convalescence, although the latter is not useful for diagnosis during the acute disease.
For epidemiologic purposes, S. aureus can be subdivided into subgroups based on the susceptibility of the clinical isolate to lysis by a variety of bacteriophages. A person carrying S. aureus of the same phage group as that which caused the outbreak may be the source of the infections.
Treatment
In the United States, 90% or more of S. aureus strains are resistant to penicillin G. Most of these strains produce β-lactamase. Such organisms can be treated with β-lactamase–resistant penicillins (e.g., nafcillin or cloxacillin), some cephalosporins, or vancomycin. Treatment with a combination of a β-lactamase–sensitive penicillin (e.g., amoxicillin) and a β-lactamase inhibitor (e.g., clavulanic acid) is also useful.
Approximately 20% of S. aureus strains are methicillin-resistant or nafcillin-resistant by virtue of altered penicillin-binding proteins. These resistant strains of S. aureus are often abbreviated MRSA or NRSA, respectively. Such organisms can produce sizable outbreaks of disease, especially in hospitals. The drug of choice for these staphylococci is vancomycin, to which gentamicin is sometimes added. Daptomycin is also useful. Trimethoprim-sulfamethoxazole or clindamycin can be used to treat non–life-threatening infections caused by these organisms. Note that MRSA strains are resistant to almost all β-lactam drugs, including both penicillins and cephalosporins. Ceftaroline fosamil is the first β-lactam drug useful for the treatment of MRSA infections.
Strains of S. aureus with intermediate resistance to vancomycin (VISA strains) and with complete resistance to vancomycin (VRSA strains) have been isolated from patients. These strains are typically methicillin-/nafcillin-resistant as well, which makes them very difficult to treat. Daptomycin (Cubicin) can be used to treat infections by these organisms. Quinupristin-dalfopristin (Synercid) is another useful choice.
The treatment of toxic shock syndrome involves correction of the shock by using fluids, pressor drugs, and inotropic drugs; administration of a β-lactamase–resistant penicillin such as nafcillin; and removal of the tampon or debridement of the infected site as needed. Pooled serum globulins, which contain antibodies against TSST, may be useful.
Mupirocin is very effective as a topical antibiotic in skin infections caused by S. aureus. It has also been used to reduce nasal carriage of the organism in hospital personnel and in patients with recurrent staphylococcal infections. A topical skin antiseptic, such as chlorhexidine, can be added to mupirocin.
Some strains of staphylococci exhibit tolerance (i.e., they can be inhibited by antibiotics but are not killed). (That is, the ratio of minimum bactericidal concentration [MBC] to minimum inhibitory concentration [MIC] is very high.) Tolerance may result from failure of the drugs to inactivate inhibitors of autolytic enzymes that degrade the organism. Tolerant organisms should be treated with drug combinations (see Chapter 10).
Drainage (spontaneous or surgical) is the cornerstone of abscess treatment. Incision and drainage (I&D) is often sufficient treatment for a skin abscess (e.g., furuncle [boil]); antibiotics are not necessary in most cases. Previous infection provides only partial immunity to reinfection.
S. epidermidis is highly antibiotic resistant. Most strains produce β-lactamase but are sensitive to β-lactamase–resistant drugs such as nafcillin. These are called methicillin-sensitive strains (MSSE). Some strains are methicillin/nafcillin resistant (MRSE) due to altered penicillin-binding proteins. The drug of choice is vancomycin, to which either rifampin or an aminoglycoside can be added. Removal of the catheter or other device is often necessary. S. saprophyticus urinary tract infections can be treated with trimethoprim-sulfamethoxazole or a quinolone, such as ciprofloxacin.
Prevention
There is no vaccine against staphylococci. Cleanliness, frequent handwashing, and aseptic management of lesions help to control spread of S. aureus. Persistent colonization of the nose by S. aureus can be reduced by intranasal mupirocin or by oral antibiotics, such as ciprofloxacin or trimethoprim-sulfamethoxazole, but is difficult to eliminate completely. Shedders may have to be removed from high-risk areas (e.g., operating rooms and newborn nurseries). Cefazolin is often used perioperatively to prevent staphylococcal surgical-wound infections.
STREPTOCOCCUS
Streptococci of medical importance are listed in Table 15–3. All but one of these streptococci are discussed in this section; S. pneumoniae is discussed separately at the end of this chapter because it is so important.
TABLE 15–3 Streptococci of Medical Importance
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


