Enzymes are biological catalysts that are involved in most biochemical reactions. As with catalysts in chemistry, they speed up chemical reactions and this is achieved by lowering the activation energy for a chemical reaction. Most enzymes are proteins, an important exception being rRNA, which has peptidyl transferase enzyme activity (Chapter 15).
Characteristics of enzymes
The proteinacious nature of enzymes means that they often show:
Activation: many enzymes are released as inactive precursors that can be activated, for example by hydrolysis, which then liberates the active enzyme. An example of this is the coagulation cascade in blood, in which the activation of a coagulation factor from its precursor involves cleavage.
pH dependence: changes in pH can affect the protein structure and function. For example, gastric enzymes such as pepsins are activated at acidic pH in the stomach but inactivated as they enter the small intestine at pH 5.3.
Temperature sensitivity: many enzymes show temperature dependence and undergo substantial increases in activity with small increases in temperature. Enzymic activity is usually optimal at the physiological temperature of 37°C. Increasing temperature can destroy the protein structure and lead to denaturation.
Enzyme kinetics
Michaelis-Menten kinetics
This is a relatively simple model that accounts for enzyme kinetics. It takes account of the substrate concentration in relation to the rate of reaction.
The Michaelis-Menten equation is summarised:
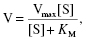
where V = rate of reaction (number moles produced per unit time), Vmax = maximum rate of reaction, [S] = concentration of substrate, KM = Michaelis constant.
The KM is defined as the concentration of substrate at which the rate of reaction (V) is half of the maximal reaction (Vmax). The KM value is typically in the range of 10−1–10−6 M, where a low concentration indicates high affinity of the enzyme for the substrate. As it is the concentration at which half of the enzyme’s active sites are occupied by substrate, it is affected by factors such as pH and temperature. The Vmax occurs when all of the active sites are occupied and the enzyme is saturated, and is therefore dependent on the quantity of enzyme present.
Figure 9.1 summarises the plot reaction for an enzyme that obeys Michaelis-Menten kinetics.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


