Fig. 23.1
a Fluorescence imaging of MSN-NH2-FITC (left) and MSN-ICG (middle) in anesthetized rats before and 90 min after i.v. injection. Scanning time was all set to be 30 s. The fluorescence image of mouse injected with MSN-ICG at 3 h p.i. was also included (right). b (Left) Excitation and emission spectra of MSN nanoprobes. Inset: the fluorescence imaging of particles which were directly acquired before (left) and after (right) excitation by Maestro imaging system. (Right) In vivo optical imaging of SLNs in a 4T1 tumor metastatic model. The tumor-drained SLNs were confirmed by bioluminescence imaging and monitored by ZW800-modified MSNs for up to 3 weeks. c In vivo optical imaging of a tumor-bearing mice after local injection of UCNP@MSNs. From left to right bright field, upconversion luminescence, and overlay images. Recreated with kind permission from Wiley–VCH Verlag GmbH & Co. KGaA [27, 50] and Elsevier B. V. [49]
Despite the success achieved by using ICG and ZW800-conjugated MSNs for optical imaging, room for further improvement is still available to minimize their inherent disadvantages, such as non-specific interactions with in vivo proteins/enzymes, low photostability, wider emission spectra [52]. Nanoparticles-based fluorescence reagents have tunable and narrow emission peaks, high quantum yields and non-photobleaching, which can serve as another promising candidate for fluorescence imaging [53, 54].
Due to the very small pore size (2–3 nm) of MSN, and to avoid potential optical quenching when decorating fluorescent nanocrystals at the surface of MSN, loading them into the mesoporous silica shell has been demonstrated to be the best way to synthesize high-quality fluorescent MSNs [25, 30]. By using cetyltrimethylammonium bromide (CTAB) as not only organic template for the formation of mesoporous silica shell, but also the stabilizing secondary surfactant for transferring the hydrophobic nanoparticles into water phase, a simple but efficient method for encapsulating any oleic acid-capped nanoparticles into mesoporous silica matrix has been developed and widely used to date [25, 26, 30]. Similar strategy has been used for the synthesis of a PEGylated liposome-coated QDs/MSN core–shell nanoparticles [40]. Although successful cellular imaging was achieved in MCF-7 breast cancer cell line by using such nanoparticles [40], so far no in vivo imaging study has been reported, possibly due to the toxicity concern from traditional cadmium-based QD [55].
In comparison with traditional organic dyes and QDs, UCNP excludes the potential ultraviolet photodamage and has been demonstrated to be much more biocompatible with extremely low toxicity in living systems [56, 57]. Besides, UCNP exhibits other attractive optical features such as sharp emission lines [58], long lifetimes (~ms) [59], large anti-Stokes shift [58], superior photostability [60], high detection sensitivity [61], non-blinking and non-bleaching [60, 62], high tissue penetration depth [63], minimal photodamage [64], and extremely low autofluorescence [65]. Moreover, by doping with well-selected lanthanide ions of Gd3+, Er3+/Yb3+, and Tm3+/Yb3+ (or combination of these ions) in suitable crystal hosts (e.g., NaYF4), magnetic resonance and multicolor upconversion luminescence (UCL) imaging could be readily achieved [62, 66–68]. With the presence of heavy metal ions like Gd3+, Yb3+, and Lu3+ in NaYF4 matrix, such UCNP has also be considered as an intrinsic computed tomography (CT) contrast agent recently [69–71]. Although UCNP@MSN could become a highly attractive candidate for cancer imaging and therapy, so far only very limited papers have been reported regarding the in vivo imaging of UCNP@MSN [27]. In one study, core–shell-structured NaYF4:Tm3+/Yb3+/Gd3+@MSN nanoparticles have been synthesized with a modified method of that reported by Kim et al. [30] and used for in vivo tumor optical and MR imaging [27]. Although only preliminary in vivo UCL imaging of NaYF4:Tm3+/Yb3+/Gd3+@MSN nanoparticles after intratumor injection has been provided (Fig. 23.1c), as-synthesized NaYF4:Tm3+/Yb3+/Gd3+@MSN could become more useful if suitable post-surface modifications, such as PEGylation, targeting ligand conjugations, and anti-cancer drug loading, could be invited in the future.
Although series of fluorescent MSNs have been developed and adopted for fluorescence imaging, most of them are still unsuitable for in vivo tumor imaging due to the limited tissue penetration depth. Also, despite NIR dyes (e.g., ICG and ZW800) conjugated MSNs or UCNP@MSN have shown promising in vivo imaging, due to the semi-quantitative nature of fluorescence imaging, engineering of MSN to enable a much more sensitive and quantitative imaging is urgently needed and will provide researchers with more accurate information about the fate of MSN in vivo.
23.3.2 PET
In PET imaging, internal radiation is administered through a low mass amount of chemicals labeled with a radioisotope. The major advantages of PET imaging over other modalities (e.g., optical and MRI) are that it is highly sensitive (picomolar detection limit) and quantitative with no tissue penetration limit [72]. Labeling nanoparticles with positron-emitting nuclides has been accepted as the most accurate mean for the non-invasive evaluation of their biodistribution [73]. Despite the above-mentioned advantages of radionuclide-based imaging, the numbers of studies using PET for MSN are rather limited.
So far, only one report about radioisotope-labeled MSN has been reported [49], where MSN-based triple-modal (fluorescence/MR/PET) nanoprobe has been developed for tracking 4T1 T-SLNs in vivo. To synthesize an MSN-based triple-modal nanoprobes (fluorescence/MR/PET), preprepared NIR dye (i.e., ZW800) doped MSN has been further labeled with gadolinium (Gd3+) or 64Cu through chelation (Fig. 23.2a). Systematic in vitro characterization and long-term (up to 3 weeks) in vivo T-SLNs mapping confirmed the high stability of as-designed nanoprobe. Based on PET imaging, the accumulation of MSNs in T-SLNs has been found to be very high and reached nearly 80 %ID/g at 1 h post-injection (p.i.), which was about 35-fold higher than that in N-SLNs (Fig. 23.2b). Although much room is still available for improving the current version of triple-modal MSN probes, the advantages for the integration of fluorescence/MR/PET together with one nanoparticle are obvious. PET imaging can be performed first to identify any skeptical “hot spots” in a highly sensitive and quantitative manner. Then, MRI will be used for achieving a better anatomical localization of site of interest. Last, on-spot optical imaging can be invited for providing an imaging guidance during surgery.
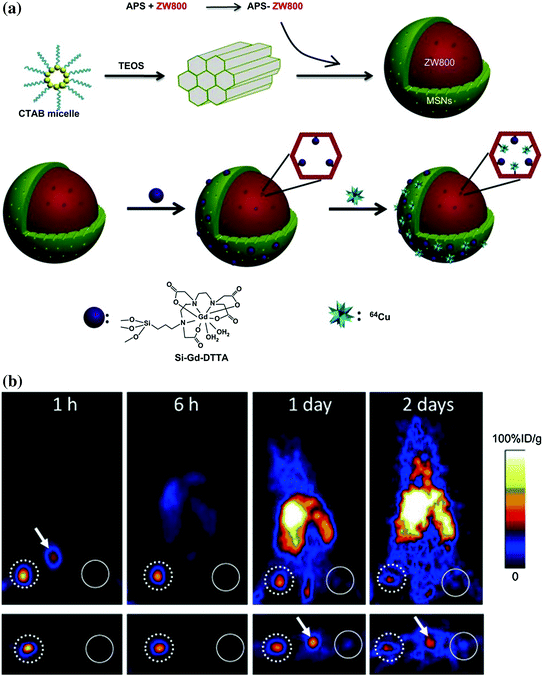

Fig. 23.2
a Schematic illustrations for the synthesis of triple-modal imaging MSN probes. (up) Diagram of ZW800-doped MSN fabrication; (down) diagram of Gd3+ and 64Cu integration. b PET imaging of SLNs with 64Cu-labeled triple-modal MSNs in a 4T1 tumor metastatic model. Recreated with kind permission from Elsevier B. V. [49]
It is worthy to know that, different from optical imaging of UCNP@MSN [27], where typically the nanoparticle itself is detected, radionuclide-based imaging detects the radioisotopes rather than MSN itself. The biodistribution of radiolabeled MSN is measured indirectly by assessing the localization of the radionuclide, which can provide quantitative measurement of the pharmacokinetics only if the radioisotope on MSN is stable enough under physiological conditions. With the possible dissociation of the radionuclide (typically metal ions) from the chelator, and/or the detachment of radionuclide-containing polymer coating from the nanoparticle, significant difference between the real nanoparticle distribution and the radionuclide distribution may occur. Thus, the biodistribution data of radiolabeled nanoparticles based on PET imaging should only always be interpreted with caution. Rigorous validation of the stability of the radioisotopes on the nanoparticle should always be carried out to obtain more reliable experimental results.
Along with the advantages of PET for sensitive and quantitative in vivo imaging, one disadvantage is that their relatively low spatial resolution (>1 mm), which is significantly lower than that of MRI (<100 μm) [74]. MRI, with exquisite soft tissue contrast, is a widely used imaging modality with high clinical relevance due to its high spatial resolution [75]. By conjugating MSN with Gd3+ complex [76] or encapsulating with varied nanoparticles (e.g., Fe3O4 [77], Gd-doped UCNP [27], MnOx [78, 79]) that can be demonstrated as MRI contrast agents, such f-MSNs could be ready to be used for MR imaging as well as drug delivery.
23.3.3 Magnetic Resonance Imaging
Generally, two major categories of f-MSNs have been developed. They are gadolinium- or MnOx– modified MSNs [78, 80–82] for T1-weighted MRI and superparamagnetic iron oxide nanoparticle (SPION)@MSN core–shell structure for T2-weighted MRI [77, 83, 84].
Gd3+-complex-modified MSNs have been used most frequently in cell tracking, especially in stem cell tracking [85–87]. In one study, dual-functional Gd-FITC mesoporous silica nanoparticles (Gd-Dye@MSN) that possess green fluorescence and paramagnetism have been developed to evaluate their potential as effective T1-enhancing trackers for human mesenchymal stem cells (hMSCs). As-synthesized Gd-Dye@MSN showed high biocompatibility with no obvious affection on the cell viability, proliferation, and differentiation capacities and has been suggested to be an ideal vector for stem cell tracking with MRI [85]. High internalizing efficiency, durability, and biocompatibility of Gd3+-complex-modified MSN have also been demonstrated in the other similar studies [86, 88]. More recently, different novel Gd3+-complex-modified MSNs have also been demonstrated useful for tumor targeting and drug delivery [83, 88, 89]. At the same time, different methods for enhancing the relativity of these MSNs are also available [76, 81, 82, 90, 91].
Manganese (II) has been adopted as an MRI contrast agent due to its less toxicity and five unpaired electrons with long electronic relaxation time. MSN-coated hollow manganese oxide (MnO) nanoparticles (HMnO@MSN) have been developed as a powerful tool for serial MR monitoring of adipose-derived mesenchymal stem cells [78], which enabled the long-term monitoring of cell transplants up to 14 days (Fig. 23.3a). Possibly due to the shielding of manganese ions by mesoporous silica coating, results showed that as-synthesized HMnO@MSN had only a very low r 1 value of about 0.99 mM−1 s−1. In another study, by using an in situ redox reaction, Chen et al. reported a novel manganese oxide-based multifunctionalization of hollow mesoporous silica nanoparticles (h-MSN@MnO) for pH-responsive T1-weighted MRI to detect the acidic tumor microenvironment [92]. Under weak acid conditions, the release of Mn2+ ions from h-MSN@MnO could cause a significant increase in r 1 value (up to 8.81 mM−1s−1), which has been demonstrated to be 11-fold higher than the same value in neutral condition. This is the highest r 1 value that has ever been reported in manganese-based MRI contrast agents, and such pH-responsive MRI contrast enhancement might become a useful technique for future cancer diagnosis.
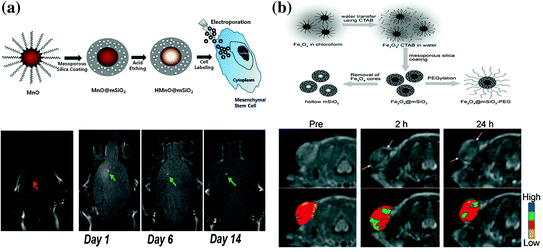

Fig. 23.3
a Up schematic illustration for the synthesis of HMnO@MSN and labeling of stem cells. Down in vivo T1-weighted MR images of transplanted stem cells. No hyperintense signal has been detected in mouse transplanted with unlabeled stem cells, while observable signals have been detected in labeled stem cells and kept visible for at least 2 weeks p.i. b Up schematic illustration of the synthetic procedure for SPION@MSN core–shell nanostructures. Down in vivo T2-weighted MR images (upper row) and corresponding color maps (lower row) of tumor before and after the i.v. injection of SPION@MSN into MCF-7 tumor-bearing nude mouse. Recreated with kind permission from American Chemical Society [78] and Wiley–VCH Verlag GmbH & Co. KGaA [77]
SPION is among the most well-developed contrast agents for T2-weighted MRI [5]. Although oleic acid-assisted strategy could guarantee high-quality SPIONs with uniform size and morphology [93], as-synthesized SPIONs are always coated with a layer of oleic acid ligands, which makes them water insoluble. Surface modifications, such as ligand exchange [94] and mesoporous silica coating [77], can efficiently overcome the hydrophobicity of original SPIONs, making them ready for biological applications. Recent research showed that the shell thickness of mesoporous silica could significantly affect the MRI contrast enhancement and might become a simple way to tune r 2 values of SPION@MSN nanoparticles [95]. With the presence of MSN at the surface, small molecular drugs [e.g., docetaxel (DOC) and doxorubicin (DOX)] can be loaded for controlled drug release and cancer therapy, suitable length of PEG could also be added to increase the blood circulation time of SPION@MSN for a better enhanced permeability and retention (EPR) effect. In one study, enhanced tumor uptake of uniform PEGylated SPION@MSN in MCF-7 tumor-bearing nude mice has been achieved based on pure EPR effect [77] (Fig. 23.3b). Higher in vivo tumor-targeting efficiency could be achieved by conjugating SPION@MSN with certain targeting ligands (e.g., proteins, peptides, or small molecules). Many other studies have further proven the feasibility in using functionalized SPION@MSN as a useful tool for tracking various types of cells with MRI [86, 95–97]. The low cytotoxicity, high cell-labeling efficiency, and high biocompatibility of SPION@MSNs make them attractive choices for a variety of biomedical applications.
23.3.4 Other Modalities and Multimodality Imaging
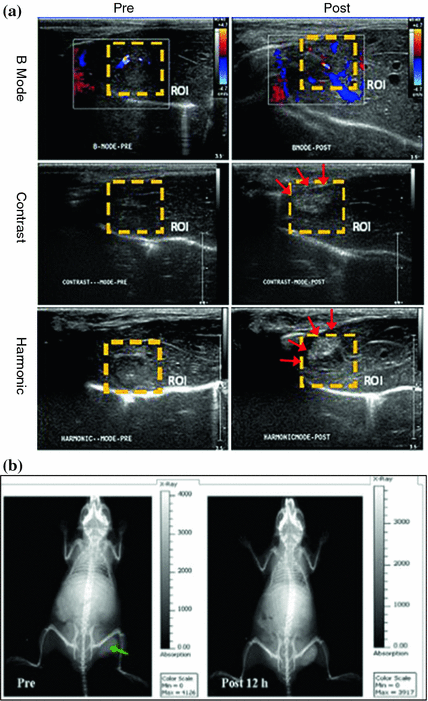
Although still under intensive investigation, f-MSNs can also be applicable in other molecular imaging modalities such as ultrasound or computed tomography (CT). Compared with other imaging modalities, ultrasound imaging stands out as a versatile, painless, low-risk, non-invasive procedure, which can be done and readily repeated virtually anywhere. Au nanoparticle and perfluorohexane-encapsulated h-MSNs have been used for both contrast-enhanced ultrasound imaging and high-intensity focused ultrasound (HIFU) surgical therapy [98, 99], demonstrating that functionalized h-MSN can serve as a novel theranostic agent for both the imaging and combined therapy under the guidance of intensified ultrasound (Fig. 23.4a). With the support of X-ray, CT can provide high-resolution anatomical information and has indisputable benefits to the practice of medicine. ICG-loaded Au@MSN has been developed recently for dual-modal fluorescence/CT imaging (Fig. 23.4b) [100]. Such probe might have great potential applications for use in applications such as cancer targeting, molecular imaging in combination with radiotherapy, and phototherapy.
Each imaging modality discussed above has its advantages and limitations. For example, it is difficult to accurately quantify a fluorescence signal in living subjects with fluorescence imaging alone, particularly in deep tissues; MRI has high-resolution and good soft tissue contrast but low sensitivity; radionuclide-based imaging techniques are very sensitive but suffer from relatively poor spatial resolution and high costs. Combination of multiple imaging modalities may provide complementary information than a single modality alone. Using MSNs as a multifunctional platform, combination with different imaging modalities will enable the development of “smart” nanomedical platforms with higher clinical potentials. One of the most selected combinations is the integration of fluorescent and magnetic materials into MSNs, which merges the high sensitivity of fluorescence with the high spatial resolution of MRI for real-time monitoring of disease evolution [27, 88] or specific cell tracking [97]. Similar rules can apply to the MSNs for CT/fluorescence dual imaging purpose [100]. Another attractive choice is the unionization of ultrasound and MRI [92]. Using MRI as the presurgical evaluation tool and ultrasound as the real-time guidance during the surgical removal of lesions, this type of MSNs can stand for the next generation of theranostic agents. MSN nanoprobe, with triple-modal (i.e., PET/fluorescence/MRI) imaging modality, has also been developed and used for long-term monitoring of tumor-draining SLNs [49]. For the multimodality imaging applications, the stability and robustness of MSNs should stay high to guarantee the imaging results from different modalities that are consistent and complementary.
23.4 Engineering of MSNs for Cancer Therapy In Vivo
Besides using MSN as a useful nanoplatform for molecular imaging, thanks to its large surface area, high pore volume, and uniform and tunable pore size, MSN has long been considered as highly attractive candidate for delivering small molecular drugs to tumor site for suppressing the growth of cancer. Despite the huge progress that has been made in the in vitro therapy with drug-loaded MSNs, in vivo translation is still facing big challenges and is now under intensive investigations. In the following sections, only engineering of MSNs for successful in vivo therapeutic studies will be highlighted.
23.4.1 Pure MSNs or h-MSNs for Chemotherapy
Although great efforts have been devoted to improve the in vivo therapeutic efficiency of anti-cancer drugs by using various kinds of drug delivery systems, developing a universal strategy for improving both the passive and active targeted drug delivery is still a huge challenge. By reducing the size of MSN to about 50 nm and further surface functionalized with a polyethyleneimine–polyethylene glycol copolymer (PEI-PEG), Meng et al. demonstrated that such redesigned MSN@PEI/PEG could effectively reduce the particle opsonization while improving the EPR effect and in vivo therapeutic effects [101]. In comparison with similar MSNs with only PEGylation, the further introduction of cationic charged PEI has been shown highly useful to enhance the repulsion between nanoparticles, which increase the particle stability and reduce the chance of their aggregation in saline (Fig. 23.5a). A significantly higher (~12 %) passive tumor-targeting efficiency of NIR dye-incorporated MSN@PEI/PEG has been demonstrated, while only limited tumor uptake has been observed in non-PEGylated (~1 %) and PEGylated (~3 %) control groups (Fig. 23.5b). Although clearly higher fluorescence signal could be seen in MSN@PEI/PEG group based on the optical imaging, due to the semi-quantitative feature of fluorescence, the real differences in EPR effect might deserve further investigation by using more quantitative method, such as PET imaging. Interestingly, no obvious kidney uptake of all three kinds of nanoparticles has been observed after 72 h post-injection, which is inconsistent with similar nanoparticle reported by the same group [102, 103]. After demonstrating the enhanced passive targeting efficiency of MSN@PEI/PEG, the therapeutic effect of DOX-loaded MSN@PEI/PEG on KB-31 tumor model has also been investigated, which showed an 85 % of tumor inhibition, 10 % higher than that of free DOX (i.e., 70 %) (Fig. 23.5c). Importantly, mice treated with DOX-loaded MSN@PEI/PEG showed reduced systemic, hepatic, and renal toxicity compared to free DOX drug. One of the possible drawbacks of these DOX-loaded MSN@PEI/PEG nanoparticles is its high lung uptake, which usually is caused by the aggregation of nanoparticle after intravenous (i.v.) injection. Although the authors claimed no significant lung damage, no reason for such high lung uptake has been provided yet.
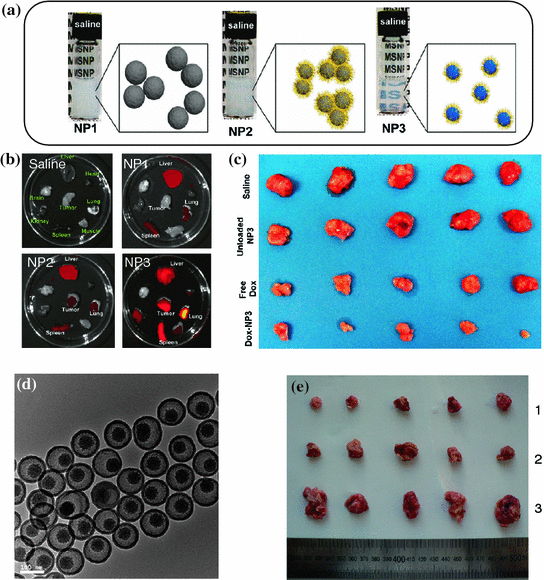

Fig. 23.5
a Photographs of three different MSNs in saline (20 mg/mL). NP1: 100-nm-sized non-PEGylated MSNs, NP2: 50-nm-sized PEGylated MSNs, NP3: 50-nm-sized PEI/PEG copolymer-modified MSNs. b Ex vivo optical imaging of major organs (heart, lung, spleen, liver, kidney, brain, and muscle) from mice at 72 h post-injection. c Photograph of tumor sizes at the end of treatment. d TEM image of SN-PEG. e In vivo anti-tumor effect of SN-PEG-DOC and Taxotere on H22 liver cancer subcutaneous model (dose: 20 mg/kg of DOC, 4 doses in 13 days). Photographs of tumors from different treatment groups of [1] SN-PEG-DOC, [2] Taxotere group, and [3] control group with saline. Recreated with kind permission from American Chemical Society [101, 102]
DOC is a hydrophobic drug which could kill cancer cells via stabilizing microtubules to induce cell cycle arrest and caspase-2- and caspase-3-dependent apoptoies [104]. The current clinical formulation of DOC, also named as Taxotere, is known to have severe adverse effect due to the acute hypersensitive reaction induced by its toxic excipients (i.e., Tween-80) and the severe systematic toxicity of DOC itself [105]. By loading DOC into a well-designed PEGylated silica nanorattle (SN-PEG) (Fig. 23.5d), Li et al. demonstrated the feasibility for enhancing the in vivo therapeutic efficiency of SN-PEG-DOC while reducing the systematic toxicity of DOC in H22 liver cancer subcutaneous model [106]. The absence of toxic Tween-80 surfactants and reduced amount of free DOC after i.v. injection have been suggested to be two of the main factors which contribute the lower systematic toxicity. Their systematic treatment experiments showed a significant inhibition of tumor weight by SN-PEG-DOC up to 72 %, in comparison with that of 57 % in Taxotere groups (Fig. 23.5e). Although no data about the passive targeting efficiency of SN-PEG-DOC in vivo could be found in this study, the author believed the possibility to further enhance such therapeutic efficiency by conjugating nanoparticle with certain targeting ligands.
23.4.2 f-MSN@PS for Photodynamic Therapy
Photodynamic therapy (PDT) has arisen as an alternative to chemotherapy and radiotherapy. PDT involves the use of certain photosensitizer (PS) which, upon irradiation with light with specific wavelength and in the presence of oxygen, could lead to the generation of cytotoxic singlet oxygen and consequently cause the killing of cancer cells [107]. Due to the limited light penetration depth, for a long time, PDT has only been effective to certain superficial cancers, such as skin cancer [107]. The combination of PDT with light in the NIR region might hold the potential for extending the treatment to tumors located deeply in body. Recent researches have demonstrated such possibility in f-MSNs either by using a well-designed PS which has high two-photo absorption (TPA) cross-section in the biological spectral window (i.e., 700–1,000 nm) [108], or by using UCNPs as an internal light transducer for transferring 980 nm laser to visible light for triggering the generation of singlet oxygen [28].
In one study, Gary-Bobo et al. reported the first example of tumor treatment by using MSNs which were covalently linked with PS and targeting ligands (mannose moiety), denoted as MSN1-mannose (Fig. 23.6a) [108]. Each MSN had about 6,850 units of PS and a hydrodynamic diameter of 118 nm. By irradiation with 760 nm laser (80 mW or 10.6 J cm−2), in vitro two-photo-excitation PDT effect showed a twofold enhanced therapeutic effect of MSN1-mannose in three cancer cell lines, in comparison with group treated with only non-targeted MSN1 nanoparticles. To investigate the potential of MSN1-mannose for in vivo treatment, nude mice-bearing HCT-116 xenografts were used. With only one i.v. injection, the authors have demonstrated a significant reduction in tumor weight (ca. 70 %) in the group treated with MSN1-mannose (dose: 16 mg/kg, light dose: 760 nm laser for three periods of 3 min) compared with control groups (Fig. 23.6b). Surprisingly, it was reported that MSN1-mannose treatment followed by two-photo-excitation PDT prevented macrometastasis formation in liver and colon, while no such effect could be observed in groups of mice treated with saline alone or with MSN1-mannose without 760 nm laser irradiation. The authors suggested the decrease in the subcutaneous tumor growth and its associated neoangiogenesis to be the main reason behind such effect. Although enhanced therapeutic effects have been demonstrated by using MSN1-mannose plus laser in this report, no data about the exact tumor uptake and biodistribution of MSN1-mannose after i.v. injection could be found.
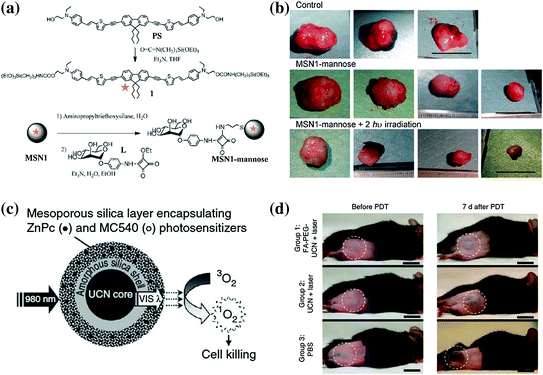

Fig. 23.6
a Synthesis of PS and MSN1-mannose. b Effect of two-photo-excitation PDT on tumor growth. c Schematic of MSN coated with UCNP coloaded with ZnPC and MC540 PSs for PDT. After the excitation of single 980 nm laser, the UCNP can convert the NIR light to visible light of different wavelength, which will be absorbed by the PSs and trigger the generation of singlet oxygen. d Representative photos of mouse injected with folic acid-conjugated PEGylated UCNP, unmodified UCNP and PBS. Scale bar: 10 mm. Recreated with kind permission from Wiley–VCH Verlag GmbH & Co. KGaA [108] and Nature Publishing Group [28]
UCNP has gained growing interests owing to their unique UCL features that are highly suitable for multimodal imaging in living subjects [11]. UCL is a unique process where low-energy continuous wave of NIR light (usually 980 nm) is converted to higher-energy light through the sequential absorption of multiple photons or energy transfer [109]. By using the visible emission bands (mostly red emissions) of UCNP for triggering the generation of singlet oxygen, researchers have developed many different versions of UCNP/PS nanocomposites for 980 nm laser-initiated PDT [28, 110–118].
In one well-conducted study, Idris et al. demonstrated that enhanced PDT effect could be achieved by encapsulating two different PSs (i.e., ZnPC and MC540) into one mesoporous silica-coated NaYF4:Er/Yb nanoparticle (Fig. 23.6c) [28]. In vitro experiments demonstrated the higher generation rate of singlet oxygen and lower cell viability when shedding 980 nm light on co-PS-loaded nanoparticles. After the injection of B10-F0 melanoma cell that prelabeled with co-PS-loaded UCNP under the skin of C57BL/6 mice, they succeeded in inhibiting the tumor growth rate significantly by irradiating the injected site with a 980 nm laser. However, in the case of injecting the drug either intratumorally or intravenously, only slight tumor growth inhibition could be achieved, indicating such in vivo PDT effect is still limited. Since the half-life of singlet oxygen is very short, on the order of microseconds, and can therefore act only on target structures that are in close proximity to it, in order to guarantee an effective PDT therapeutic outcome, sufficient amount of PDT agents should be able to accumulate in the tumor site and somehow further internalized by the cells. Much room is still available for enhancing the UCNP-based PDT therapeutic effect in the future.
23.4.3 MSN for Gene Delivery and Therapy
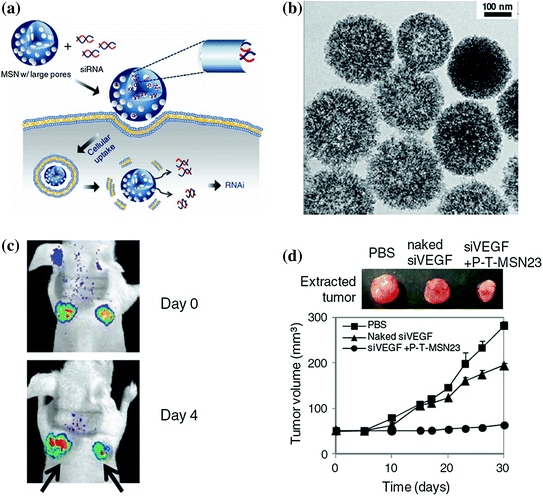
Although small interfering RNA (siRNA) holds great promising as a powerful gene therapeutic agent for the treatment of a wide range of diseases from viral infection to cancer [119, 120], it is still a big challenge to deliver effective dose of siRNA into target cells with high gene-silencing efficacy while maintaining its intact chemical structure. Aiming to provide a better solution to these problems, except using previously reported nanoplatforms, such as viral gene or liposomes, Na et al. reported a highly efficient siRNA delivery system based on biocompatible MSNs with large pores of 23 nm in diameter (denoted as MSN23) and have demonstrated their advantages in siRNA delivery for gene silencing both in vitro and in vivo (Fig. 23.7a) [121]. As-designed MSN23 was prepared by expanding the original pores of MSN2 (mesoporous silica with 2 nm pore size) with trimethylbenzene (Fig. 23.7b). With the presence of larger pores, they have demonstrated that siRNA could be loaded into the MSN23 with a decent loading capacity of 1.25 pmol/μg and shown high resistance against the RNase-mediated degradation while maintaining its chemical integrity in vivo. The in vivo siRNA delivery and gene silencing by using siRNA–MSN complex have been demonstrated in mice-bearing green fluorescent protein (GFP) expressing HeLa and MDA-MB-231 xenografts, which showed significantly decreased green fluorescence intensity and inhibition of tumor growth rate in positive groups after intratumoral injection with siGFP (or siVEGF)-MSN complexes vascular endothelial growth factor (VEGF) (Fig. 23.7c, d). Although the intratumoral administration of siRNA-MSN in this study, to a certain degree, limited its application and needed further improvement by optimizing the surface modifications, it ensured the accumulation of sufficient siRNA in tumor site and guaranteed the efficiency in gene silencing.