Fig. 33.1
A famous example of neutrophil chemotaxis and phagocytosis. The neutrophil is seen migrating toward a bacterium as it secretes some kind of unknown chemoattractant (e.g., N-formyl peptides like fMLP). The neutrophil polarizes its cytoskeleton and extends lamellepodia toward an increasing concentration of proteins that guide the cell toward the bacterium. Note the ability of the neutrophil to rapidly change direction and polarization in response to movement of the bacterium, as well as its ability to navigate around red blood cells. In its final fate, the bacterium is engulfed by the neutrophil and killed during phagocytosis. (The above images were taken from a 16 mm video recorded by David Rogers of Vanderbilt University in the 1950s. Details can be found at www.biochemweb.org/neutrophil.shtml)
Neutrophil chemotaxis can be broken down into three distinct but overlapping stages: chemosensing, polarization, and locomotion (reviewed in [44, 45]). When a neutrophil senses a concentration gradient of external secreted proteins known as chemokines, the cell polarizes its cytoskeleton and engages in sustained directional migration toward the attractant molecules (i.e., chemoattractant). We will provide a brief overview of the biology of chemotaxis for neutrophils and refer the reader to the aforementioned reviews for a more in-depth treatment of the subject.
33.2.1.1 Chemosensing
Neutrophils stay in an inactive and immobile state until they are activated by external cues. When a chemoattractant is present, neutrophils extend and retract lamellipodia—protrusions of the cell membrane comprised of actin filaments—to dynamically form pseudopods for about 60s intervals. If a uniform concentration of chemoattractant is present, neutrophils migrate more or less randomly until the cell senses a gradient of chemoattractant and begins to migrate directionally. Neutrophils sense chemoattractants using transmembrane chemoattractant receptors (e.g., fMLP-R and C5a-R) that become occupied by extracellular chemokines, and these receptors activate heterotrimeric G-proteins that transduce the extracellular signals and initiate an intracellular activation cascade. The chemoattractant receptors are relatively uniformly distributed on the cell membrane, and the local and temporary nature of the pseudopod formation allows neutrophils to rapidly change the extension of pseudopodia in reaction to location changes of the source of chemoattractant. Once the cell becomes polarized, neutrophils maintain the original leading edge of the cell and turns toward the new source of chemoattractant rather than forming new pseudopodia.
33.2.1.2 Polarization and Locomotion
Neutrophils can become polarized in a uniform concentration of chemoattractant; however, polarization is much more likely when a concentration gradient exists, resulting in persistent migration toward the source of chemoattractant (chemotaxis). When there is a difference as little as 1–2 % in the number of receptors on opposite ends of the cell occupied by chemoattractants, the neutrophil polarizes its cytoskeleton by increasing the number of advancing pseudopodia on the anterior portion of the cell and enriching retracting uropods with myosin in the posterior portion of the cell. Once activated and polarized, neutrophils have the ability to persistently migrate toward a site of inflammation that is sending inflammatory signals (chemokines) or chase down pathogens such as bacteria to phagocytose and clear the pathogen. The persistent migration of neutrophils is determined by a variety of factors such as type of chemoattractant, steepness, and profile of the concentration gradient and other biophysical and biochemical factors in the neutrophils’ extracellular microenvironment.
33.2.2 Chemotaxis in Disease
In the introduction, we described chemotaxis as central to the pathophysiology of many diseases. Indeed, chemotaxis is relevant to the spread of metastatic cancer [36], wound healing [33, 46, 47], the inflammatory response by the innate immune system [48, 49], rheumatoid arthritis [50], chronic obstructive pulmonary disorder (COPD [51]), and asthma [52–54]. The role of chemotaxis in the pathogenesis of these and many other diseases has been intensely studied over the last 50 years. Researchers have made progress in identifying chemotactic cell types central to the pathogenesis of a disease, as well as the identification of cytokines orchestrating cell mobility.
For example, the dissemination of metastatic cancer cells from a primary tumor site throughout the body is central to the pathophysiology of the disease. If a primary tumor is detected early, treatment regimens and prognosis are greatly improved compared to late-stage detection [55]. In other words, the cancer becomes much harder to treat once the cells have migrated away from the primary tumor site, entered the bloodstream (after angiogenesis has been initiated), and extravasated from the blood stream to form a secondary tumor. If the cancer cells have not left the primary tumor site, the cancer can often be surgically removed or treated with local irradiation. Although directional migration is not necessary for this process, there is evidence that metastases occur more efficiently when the cells migrate directionally [56]. Researchers have also shown that the chemotaxis of tumor cells toward macrophages is important for intravasation into blood vessel and for invasion into the tissue of a secondary site [57]. Minimizing or disrupting the ability of cancer cells to undergo chemotaxis may lead to a reduction in the metastasic potential of cancer cells, resulting in improved prognostic outcomes for cancer patients.
In asthma, the dysregulation of inflammatory response contributes to the pathogenesis of the syndrome. Asthma is a condition characterized by chronic inflammation of the lungs that ultimately leads to obstruction of airflow, resulting in clinical symptoms including wheezing, coughing, and shortness of breath. The condition has far-reaching impact and affects more than 300 million people worldwide [58]. Furthermore, asthma prevalence has increased significantly over the last 30 years in many regions, with some indications that it may be reaching a plateau in the developed world [52, 59]. Researchers have made progress uncovering the mechanisms and pathophysiology of asthma, leading to improved treatment and management. However, diagnosing asthma still remains a challenge for physicians [60–62], and misdiagnosis can lead to unnecessary treatment, greatly increased medical costs [63], or missed treatment for vulnerable populations such as the elderly [61, 62]. In this case, mitigating the hyperchemotactic activity of inflammatory cells could lead to improved clinical outcomes for asthmatic patients.
Cancer metastasis and asthma represent two diseases in which chemotaxis can play a central role in the pathophysiology of disease. Researchers have studied these pathways in an attempt not only to understand the biology of a disease, but also to develop targeted therapies. For instance, a therapy blocking the production of interleukin-5 (IL-5)—a cytokine that is implicated in mucus secretion, the recruitment of eosinophils to the lungs (leading to eosinophilia for asthmatic patients), and airway hyper-responsiveness (AHR)—might decrease eosinophil and mast cell recruitment to the lungs, resulting in symptom relief for the patient [64]. Anti-IL-5 clinical trials are currently underway [65, 66], demonstrating how therapeutic interventions can be developed once the underlying pathway is uncovered. In order to understand the role of cell chemotaxis in various diseases and facilitate the development of drug therapies, researchers require robust in vitro assays to controllably and systematically study cell chemotaxis. In the following sections, we will explore both traditional macroscale chemotaxis assays and more recently developed microfluidic systems. Furthermore, we will explore how properly designed microfluidic chemotaxis devices can potentially be used in a clinical setting for diagnostic and therapeutic purposes.
33.2.3 Traditional Methods for Studying Cell Chemotaxis
Several assays have been developed to study cellular chemotaxis (Fig. 33.2). The first widely adopted in vitro assay was developed by Stephen Boyden in 1962 [67], known as the “Boyden chamber” or “Transwell” assay. The Transwell assay is a two-chamber system, with each chamber filled with media and separated by a microporous membrane (Fig. 33.2a). The lower chamber contains a test substance of interest (e.g., chemoattractants), and the upper chamber contains cells. During the course of an experiment, the source compound diffuses across the porous membrane due to the concentration gradient that exists between the two chambers. Chemotactic cells sense the spatial distribution of chemoattractant and, depending on the cell type and chemoattractant, migrate across the membrane and toward the source. Multiple wells can be studied simultaneously and in parallel, allowing incorporation of proper controls (i.e., no concentration gradient) or to test varying doses in order to characterize the chemotactic response of the cell type–compound combination. Several early studies demonstrated the utility of having an in vitro chemotaxis assay by characterizing the chemotactic response of several cell types under various experimental conditions [67, 71–73]. Following the successes of the Boyden chamber, researchers sought to develop new designs that offered different approaches to measuring cell chemotaxis.
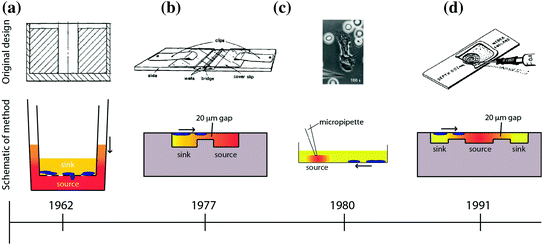

Fig. 33.2
Overview of macroscale chemotaxis assays that have been developed over the years. The timeline below each assay indicates the date the technology was invented below each assay (not to scale). The direction of the arrow indicates the direction of cell chemotaxis. The color shading indicates the formation of the chemical gradient of chemoattractant [67–70]. a Boyden/Transwell. b Zigmod. c Micropipette/Needle. d Dunn
Alternative in vitro chemotaxis assays that have been utilized in the biology community include the Zigmond chamber [68]; the Dunn chamber, which is a modification of the Zigmond chamber design [70]; the under-agarose assay [74]; and micropipette-based assays [69]. A comparison of some of these chemotaxis techniques is shown in Fig. 33.2. The Zigmond chamber consists of two etched wells—a chemoattractant (source) well and a well containing cells and media (sink)—that are separated by bridge that restricts convection of fluid from one well to the other (Fig. 33.2b). The cells are placed on an inverted coverslip spanning the two wells, allowing the investigator to observe cellular chemotaxis across the bridge in response to a chemical gradient. However, the gradients are short-lived (~1 h) and are somewhat unstable due to the evaporation that occurs in this design. The Dunn chamber design [70] sought to overcome these disadvantages and employs a spherical geometry, allowing the wells to be completely encapsulated by the coverslip to limit evaporation (Fig. 33.2d). The under-agarose assay is another early example of a technique that allowed the investigator to visually observe the migration of cells over time. In this assay, holes are punched in agarose gel, and a chemoattractant is placed in the holes to act as a source for the concentration gradient. Due to the concentration gradient between the source and the surrounding gel, the compound diffuses into the gel and cells sense the changing spatial distribution of the source compound over time and migrate toward the source under the agarose gel. This method is a simple and robust technique for creating one or more chemical gradients, while visualizing the response of cells in a physically confined environment. Foxmann et al. utilized the multisource capabilities of the under-agarose assay to characterize the neutrophil chemotactic function in competing gradients [75], and there are many other studies that utilize this technique [76, 77]. However, as useful as these in vitro chemotaxis assays have been over the years, researchers have discovered several limitations that limit their utility.
Technological limitations of these assays include the following: large sample volume requirements, unstable or unpredictable gradient profiles, and usability issues that make it difficult to run the desired experiment. For example, the most common macroscale chemotaxis assay in use today—the Transwell assay—develops unstable chemical gradient profiles [78]; filters cells by size and deformability due to the rigid microporous membrane the cells must cross [79]; and delivers ambiguous results as chemotaxis and chemokinesis are difficult to differentiate in this system [80]. The under-agarose assay allows for visualization of the cell migration path, but produces an unstable chemical gradient in all directions [78]. Additionally, under-agarose assays have low spatial resolution with respect to source and cell placement because of the way the experiment is conducted. The user is required to define these locations with a micropipet and crude hole-punching methods for the source of chemoattractant, both of which can be subject to significant variability. Therefore, while the method is relatively simple to perform and can be useful for visualizing cell migration paths, the under-agarose assay is not well-suited for reliable quantification of cell chemotaxis. Another popular and more modern visual chemotaxis assay is the Micropipette assay (also known as the “Needle assay”). This technique offers significant technical advances over these older techniques, such as high-resolution real-time imaging (Fig. 33.2c and [81]). However, the method is also best suited for qualitative analysis because its onerous labor requirements limit investigators to low-throughput sampling. Another significant limitation of most macroscale assays is that they require large sample volumes in order to isolate specific cell types of interest. For example, to perform a neutrophil chemotaxis experiment using these common macroscale techniques, the sample preparation includes a blood draw and cell purification protocols [82] requiring tens of milliliters of blood. This sample volume requirement limits the number of times a subject can be sampled to probe their neutrophil chemotactic function, thereby restricting the time resolution of the experiment. Additionally, the large blood volumes needed makes sampling infants or small animals (e.g., mice, rats, etc.) for neutrophil chemotaxis logistically complex, if not impossible.
Microfluidic technologies play an important role in providing solutions to many of these limitations. Indeed, over the last decade, microfluidic engineers have either solved or minimized the gradient stability and sample volume limitations of macroscale assays. What properties of microfluidic systems enable them to improve upon current chemotaxis assays? In the following sections, we will detail why microfluidic technologies are particularly useful for chemotaxis assays by exploring the physics of fluids at the microscale.
33.2.4 Microfluidic Methods for Studying Cell Chemotaxis
Microfluidic devices are ideally suited to perform chemotaxis assays and offer significant advantages over macroscale approaches. As previously described, chemotactic eukaryotic cells can sense spatial and temporal perturbations in their microenvironment, and respond by directionally migrating toward the increasing concentration of the stimulus. The cell cannot undergo chemotaxis, however if the concentration changes so suddenly that the receptors on the cell membrane are unable to discern a difference in soluble factors from one side of the cell to the other. Fortunately, the physics of fluids at the micro/nanoliter scale makes creating diffusion dominant mass transport a relatively trivial task. Fluids at this scale are often characterized by a series of dimensionless quantities [83]; the quantity that is most widely cited is the Reynolds number (Re, Eq. 33.1):
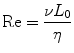
where v is the characteristic velocity of the fluid; L 0 is the characteristic length scale in which the fluid is traveling; and η is the kinematic viscosity of the fluid. For devices with dimensions on the micrometer scale that manipulate fluids with typical velocities (v ≪ m/s) and kinematic viscosity (e.g., aqueous solutions, oils, etc.), the Reynolds number, Re ≪ 1, is well under the transition from turbulent to laminar flow. Thus, the size scale of microfluidic technologies dictates that fluids generally behave according to the laminar flow regime. The implications of this phenomenon as applied to creating controllable gradients of soluble proteins are significant. Laminar fluids can be manipulated, combined, and separated in highly predictable and reproducible ways without the introduction of stochastic convective currents (turbulence). Specifically, two or more neighboring microfluidic streams can run adjacently to each other without turbulent mixing; instead, molecular diffusion dominates the mass transport between the laminar fluids.
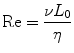
(33.1)
Diffusion is a process governed by Brownian motion, where molecules move from a higher concentration to a lower concentration (i.e., thermodynamically driven). The kinetics of this process are determined by the activation energy required to move the diffusing molecule (e.g., proteins) through a particular medium (e.g., aqueous solution). In one dimension, diffusion can be modeled by (Eq. 33.2):
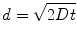
where d is the distance a molecule with diffusivity D travels in time t. There is another dimensionless quantity that describes the ratio of convection of a fluid compared to diffusion of a molecule within that fluid, known most commonly as the Peclet number (Eq. 33.3):
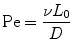
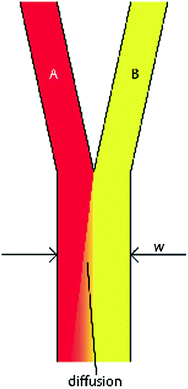
where v and L 0 are the characteristic velocity of the fluid and length scale of the system, respectively, and D is still the diffusivity of a molecule from one fluid into the medium of an adjacent fluid. This number helps to determine the relative importance of diffusion compared to the convection of the fluid. In practice, the Pe number can be used to determine the necessary length of a microchannel to allow a complete diffusion of a molecule from one fluid stream to another. For example, consider two fluids—Fluid A and Fluid B—flowing in adjacent laminar flow streams at 10 μm/s in a microchannel with width of 500 μm, and we wish to calculate the channel length required for a protein with diffusivity of 50 μm2/s to diffuse from Fluid B to the channel edge of Fluid A (Fig. 33.3). Due to the lack of convective mixing on this scale, the protein B will take 20 channel widths or 5 cm to diffuse across Fluid A (a distance a few times the diameter of a human hair). These numbers demonstrate that diffusion can be well controlled within microchannels, but without convection, transport of molecules from one area of a microchannel to another takes considerable time.
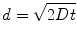
(33.2)
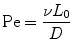
(33.3)

Fig. 33.3
Generic design of two fluids flowing through a microchannel in the laminar flow regime. Diffusion of molecules from fluid B occurs transverse to the fluid flow and down the concentration gradient toward fluid A
Microfluidic engineers have leveraged the unique properties of fluids at the microscale to make significant advancements in the capabilities of chemotaxis assays. Microfluidic devices are ideally suited tools for chemotaxis assays because they can simulate the diffusion-dominant phenomena present in the cellular microenvironment. A classic example of a microfluidic chemotaxis device that leverages microfluidic fluid phenomena is the “Christmas tree” design developed by George Whitesides’ lab in 2000 [13]. In this system, multiple concentration gradients are created by mixing fluid streams with different source concentrations in serpentine microchannels (Fig. 33.4a), then recombining the fluids with different combinations of the source concentration once the serpentine channels meet downstream. The result is a user-defined concentration gradient of the original source molecules (Fig. 33.4b). This channel design exploits laminar flow phenomena to generate highly reproducible concentration gradients that are difficult or impossible to achieve using macroscale techniques. The laminar flow properties of the system allow for the components of each source fluid to be separated and then recombined in a predictable and controllable fashion. However, the lack of convection in the system means a serpentine channel design must be utilized to achieve complete mixing due to the high Peclet number. This channel design has been used for developing chemical gradients for multiple cell types, including neutrophils [13], neural stem cells [84], Dictyostelium discoideum [85], and metastatic breast cancer cells [86].
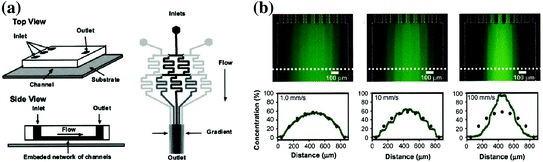

Fig. 33.4
Schematic and data of the microfluidic “Christmas tree” gradient generator. a Design of the serpentine gradient generator that shows the splitting and recombination of multiple fluid streams to create a chemical gradient. b Gradient profiles of fluorescent dye that show the versatility of the technique [88]
The “Christmas tree” design is one example of leveraging microfluidic phenomena to develop highly predictable chemical gradients; however, many other gradient devices have been developed over the years using microfluidics. For example, Wong et al. describe a microfluidic system that utilizes hydrogel barriers to compartmentalize the source, sink, and cell channels [87].
Here, the authors make use of properties of the hydrogel that enable its use in cell culture—namely the high viscosity of the hydrogel that prevents convective mass transport and the permeability of hydrogels, allowing protein diffusion from one compartment to another (e.g., from the source, to the cells, to the sink). In another class of static, no-flow microfluidic gradient generators, engineers have taken advantage of the greatly increased resistance to fluids as microchannel height is decreased:
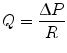
where Q is the flow rate of the fluid, ∆P is the pressure drop across the microchannel, and R is the fluidic resistance of the microchannel. Equation 33.4 shows that the convection rate of the fluid is inversely proportional to the resistance in of the microchannel. For a microchannel with rectangular cross section and high aspect ratio (width of channel far greater/less than the height of the microchannel), the resistance in the microchannel is given by:
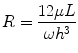
where h and w are the height and width of the channel, respectively; L is the length of the microchannel; and μ is the viscosity of the fluid [89]. From Eq. 33.5, we see that the resistance of a microchannel with a high aspect ratio is inversely proportional to the cube of its height. Therefore, a device can be engineered to be highly resistive to convection by decreasing the height of the microchannel (e.g., 7–15 μm) compared to the height of the cell, source, and sink channels. This technique of separating microchannels containing cells and reagents by low-height “diffusion channels” has been exploited to create no-flow gradient devices [90, 91] and for microfluidic multiculture systems [92]. This approach offers the benefits of highly controlled chemical gradients without requiring tubing or active pumping systems and conserves intercellular signaling that would normally be lost in a convection-based design. Other microfluidic designs have been reported that emphasize user-friendly operation to facilitate adoption by biologists, while generating robust chemical gradient profiles [93]. For example, our lab recently reported a two-component chemotaxis device that sorts neutrophils within minutes and can easily form a chemical gradient by placing a lid with chemoattractant onto a base where the neutrophils have been sorted [30]. This technique enabled a new set of applications that were difficult or impossible to conduct using macroscale chemotaxis assays, demonstrating how microfluidic systems can not only simplify, but also enhance the capabilities of biomedical researchers. The technological advancements in microfluidics combined with their demonstrated advantages over macroscale assays lead to another potential application, which is clinical diagnostic chemotaxis assays.
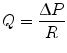
(33.4)
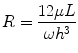
(33.5)
33.2.5 Microfluidic Chemotaxis for Translational Research
Assays that directly assess cellular chemotactic function have the potential to make valuable contributions to medicine. The concept of probing the function of a cell type to confirm disease state is beginning to show promising results. This approach seeks to elicit information from the cell type of interest in order to diagnose disease, or perhaps monitor drug therapies. However, the approach of using cell chemotaxis as a readout for clinical medicine is at an embryonic stage, with few studies demonstrating this approach to-date. The few existing published studies share several characteristics, including superior control of the biochemical gradient compared to macroscale chemotaxis assays; user-friendly operation helped by utilizing passive pumping techniques [94] for fluid handling and the use of visual readouts that track cell migration over time.
Neutrophils are a possible candidate cell type for eliciting disease information based on chemotaxis function because of their central role in the pathophysiology of several diseases, as well as their robust ability to undergo chemotaxis. Several studies have probed neutrophils for diagnostic information [90, 91, 95]. For example, Butler et al. described a neutrophil chemotaxis platform that analyzed neutrophils from burn patients to characterize their health status [91]. In this study, the authors found that the magnitude of the burn injury negatively correlated with the speed of neutrophil migration toward the chemoattractant and impaired neutrophil chemotaxis observed as early as 24 h after the patient was burned. This result validates trends observed in previous studies using traditional chemotaxis techniques, such as the Transwell and Zigmond assays [96], although those studies did not document changes in neutrophil function until 72 h after the patient was burned. Additionally, the authors found a correlation between total body surface area of the burn and neutrophil chemotaxis function of the patient. The improved detection of disease phenotypes for the burn patients compared to macroscale techniques is likely due to the improved sensitivity that microfluidic gradient generators can achieve. Keeping user-friendly design considerations in mind, the authors designed this microfluidic chemotaxis device to operate without any external fluid handling equipment (Fig. 33.5a). In contrast, most traditional macroscale chemotaxis assays would be difficult to operate in a clinical setting. This study demonstrates how microfluidic assessment of chemotactic function can provide rich, functional information that can potentially be used as a diagnostic, prognostic, and/or therapeutic biomarker.
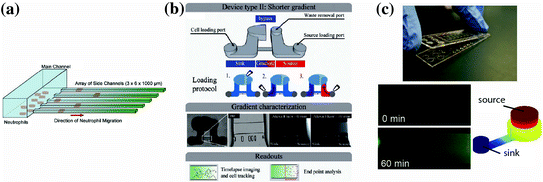

Fig. 33.5
Recent user-friendly chemotaxis devices that have been utilized for clinical applications. a Neutrophil chemotaxis through cell-sized microchannels (3 μm × 6 μm) that allow for easy quantification, robust persistent neutrophil chemotaxis, and percent convection from the source to the sink [91]. b A chemotaxis device that also utilizes a low-height “diffusion channel” to prevent convection, along with a low-resistance “bypass” to direct any potential convection in the diffusion channel [90]. c A neutrophil chemotaxis device that operates by placing a lid containing chemoattractant onto a base containing the cells. Once the connection is made, the chemoattractant can diffuse into the microchannel where the cells are visually tracked over time. Experimental and modeling data of the gradient development are shown. Importantly, all three of these methods can be operated using only a micropipette, and do not require complex fluid handling equipment of expertise [30]
In our research lab, we have also explored the possibility of using neutrophil chemotaxis for diagnostic applications [90]. Berthier et al. describe a microfluidic chemotaxis device that can easily establish biochemical gradients in a high-throughput screen and automatically track neutrophil chemotactic function (Fig. 33.5b). This study analyzed the neutrophil chemotactic function from an infant who presented recurrent bacterial infections. The analysis showed a significantly retarded neutrophil chemotactic response for the patient compared to both healthy controls and the infant’s parents (0.7 μm/s vs. 0.15–0.17 μm/s, respectively). The assay enabled analysis of changing neutrophil morphology and quantitative characterization of the neutrophil chemotaxis. Importantly, both pieces of information conveyed a signaling defect for the patient. Indeed, the patient was later diagnosed with a rare genetic mutation in a GTPase Rac2 (D57 N) that has been previously reported in the literature for an infant with immunodeficiency [97]. This sort of rare immunodeficiency is difficult to diagnose clinically, but this study demonstrates that a functional readout based on cell chemotaxis can potentially aid in making the diagnosis. In another clinical study, we are employing an adaptation of a previously published chemotaxis platform (Fig. 33.5c, [30]) to study whether neutrophil chemotaxis can be used as a biomarker to characterize or diagnose asthma. In this study, we have utilized a microfluidic kit design, where all the reagents required to run the assay are assembled in a complete, user-friendly assay. Additionally, the method employs a neutrophil sorting technique [93] that can be performed in several minutes using blood obtained from a lancet puncture. These features make it simple to rapidly perform the chemotaxis assay and make the system well suited for implementation in a clinical setting. Preliminary results from this study indicate that neutrophil chemotactic function may be impaired for asthmatic patients compared to non-asthmatic, allergic rhinitis patients. Although the results still need to be confirmed with additional experiments, the study illustrates how microfluidic solutions can provide significant advantages over macroscale assays for clinical applications. Furthermore, these reports demonstrate that chemotaxis can be a useful readout in the clinic to assist physicians with diagnosis or management of a variety of diseases.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


