INJECTABLE PREPARATIONS WITH CLINICAL APPLICATIONS
Objectives
• Select the correct syringe and needle for a prescribed injectable drug.
• Explain the procedure for preparing and calculating medications in powder form for injectable use.
• Determine prescribed insulin dosage in units using an insulin syringe.
• Explain the various methods of insulin administration.
• State the various sites for intramuscular injection.
• Explain how to administer intradermal, subcutaneous, and intramuscular injections.
Medications administered by injection can be given intradermally (under the skin), subcutaneously (SubQ, into fatty tissue), intramuscularly (IM, into the muscle), and intravenously (IV, into the vein). Intravenous injectables are discussed in Chapter 10. Injectable drugs are ordered in grams, milligrams, micrograms, grains, or units. Preparations of injectable drugs may be packaged in a solvent (diluent or solution) or in a powdered form.
This chapter is divided into five sections: (1) injectable preparations, such as vials, ampules, syringes, needles, and pre-filled cartridges; (2) intradermal injections; (3) subcutaneous injections, including heparin; (4) insulin injections; and (5) intramuscular injections from prepared liquid and reconstituted powder in vials and ampules and the mixing of drugs in a syringe. Examples and practice problems follow each section, and the answers to the practice problems are located at the end of the chapter.
Note: Answers should be rounded off in tenths or whole numbers.
INJECTABLE PREPARATIONS
Vials and Ampules
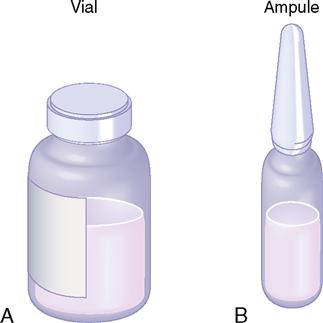
Drugs are packaged in vials (sealed rubber-top containers) for single and multiple doses and in ampules (sealed glass containers) for a single dose. Multiple-dose vials can be used more than once because of their self-sealing rubber top; however, ampules are used only once after the glass-necked container is opened. The drug is available in either liquid or powder form in vials and ampules. When drugs in solution deteriorate rapidly, they are packaged in dry form and solvent (diluent) is added before administration. If the drug is in powdered form, mixing instructions and dose equivalents such as milligrams (mg) per milliliter (mL) are usually given; if not, check the drug information insert. After the dry form of the drug is reconstituted with sterile water, bacteriostatic water, or saline solution, the drug must be used immediately or refrigerated. Usually, the reconstituted drug in the vial is used within 48 hours to 1 week; check the drug information insert. A vial and an ampule are shown in Figure 9-1.
The route by which the injectable drug can be given, such as SubQ, IM, or IV, is printed on the drug label.
Syringes
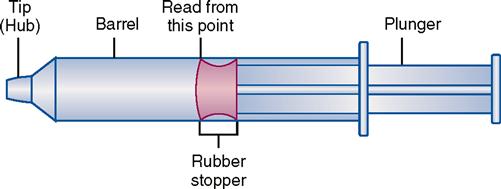
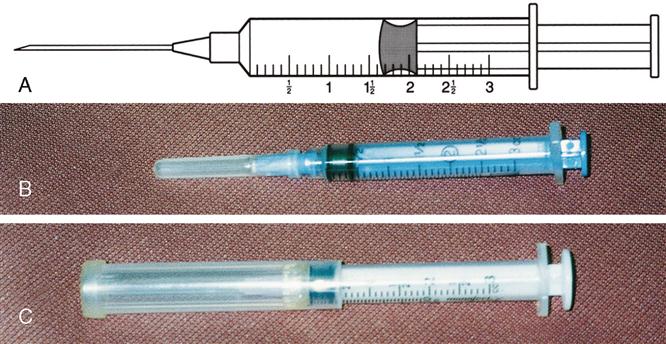
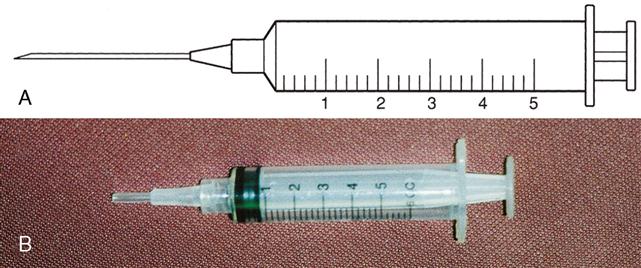
Types of syringes used for injection include 3-mL and 5-mL calibrated syringes, metal and plastic syringes for pre-filled cartridges, tuberculin syringes, and insulin syringes. There are 10-mL, 20-mL, and 50-mL syringes that are used mostly for drug preparations. A syringe is composed of a barrel (outer shell), a plunger (inner part), and the tip, where the needle joins the syringe (Figure 9-2).
Three-Milliliter Syringe
The 3-mL syringe is calibrated in tenths (0.1 mL). The amount of fluid in the syringe is determined by the rubber end of the plunger that is closer to the tip of the syringe (Figure 9-3). An advance in safety needle technology is the SafetyGlide shielding hypodermic needle (Figure 9-4). The purpose of this type of needle is to reduce needlestick injuries.
Five-Milliliter Syringe
The 5-mL syringe is calibrated in 0.2 mL increments. A 5-mL syringe usually is used when the fluid needed is more than 2½ mL. This syringe is frequently used to reconstitute a dry drug form with sterile water, bacteriostatic water, or saline solution. Figure 9-5 shows the 5-mL syringe and its markings and the 5-mL needleless syringe.
Tuberculin Syringe
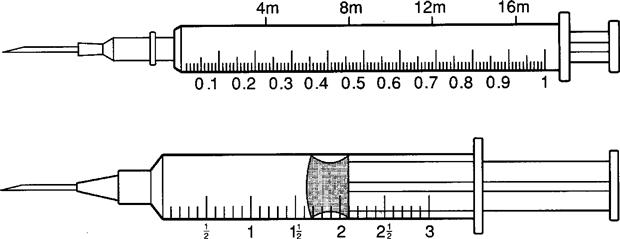
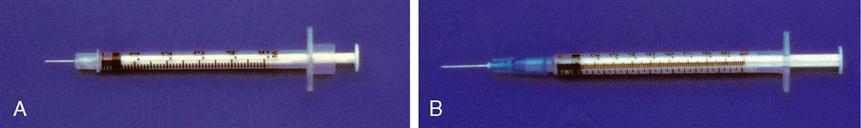
The tuberculin syringe is a 1-mL slender syringe that is calibrated in tenths (0.1 mL), hundredths (0.01 mL), and minims (Figure 9-6). This syringe is used when the amount of drug solution to be administered is less than 1 mL and for pediatric and heparin dosages. The tuberculin syringe is also available in a ½-milliliter (mL) syringe. Figure 9-7 shows the ½-mL and the 1-mL tuberculin syringes.
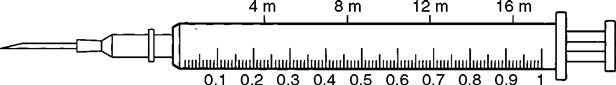
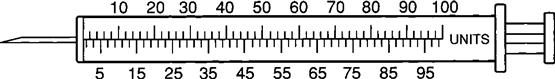
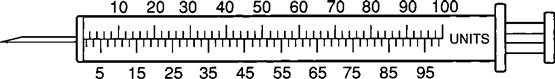
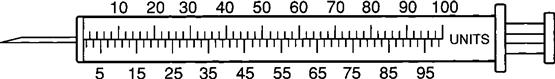
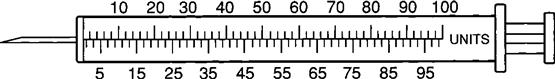
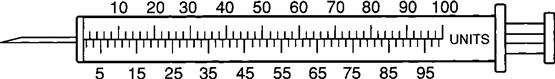
Insulin Syringe
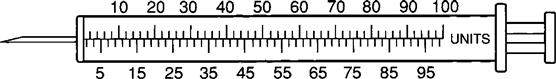
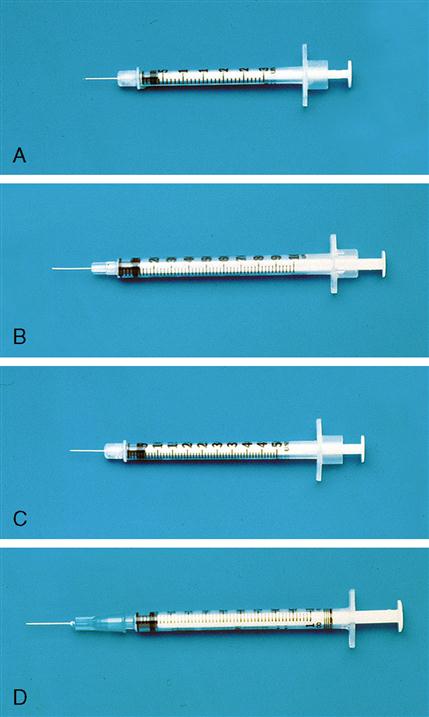
The insulin syringe has a capacity of 1 mL. Insulin is measured in units, and insulin dosage must NOT be calculated in milliliters. Insulin syringes are calibrated as 2 units per mark, and 100 units equal 1 mL (Figure 9-8). Insulin syringes, NOT tuberculin syringes, must be used for administering insulin. Insulin syringes are available in 3/10-mL, ½-mL, and 1-mL sizes. The 1-mL insulin syringe may be purchased with a permanently attached needle or detachable needle (Figure 9-9).
Pre-Filled Drug Cartridge and Syringe
Many injectable drugs are packaged in pre-filled disposable cartridges. The disposable cartridge is placed into a reusable metal or plastic holder. A pre-filled cartridge usually contains 0.1 to 0.2 mL of excess drug solution. On the basis of the amount of drug to be administered, the excess solution must be expelled before administration. Injectables are also supplied by pharmaceutical companies in ready-to-use pre-filled syringes that do not require a holder. Figure 9-10, A, shows a Carpuject syringe. Figure 9-10, B, shows a Tubex syringe.
Needles
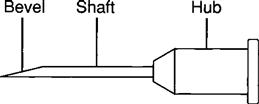
A needle consists of (1) a hub (large metal or plastic part attached to the tip of the syringe), (2) a shaft (thin needle length), and (3) a bevel (end of the needle). Figure 9-11 shows the parts of a needle.
Needle size is determined by gauge (diameter of the lumen) and by length. The larger the gauge number, the smaller the diameter of the lumen. The smaller the gauge number, the larger the diameter of the lumen. The usual range of needle gauges is from 18 to 26. Needle length varies from ⅜ inch to 2 inches. Table 9-1 lists the sizes and lengths of needles used in intradermal, subcutaneous, and intramuscular injections.
TABLE 9-1
| Type of Injection | Needle Gauge | Needle Lengths (inch) |
| Intradermal | 25, 26 | 3/8, 1/2, 5/8 |
| Subcutaneous | 23, 25, 26 | 3/8, 1/2, 5/8 |
| Intramuscular | 19, 20, 21, 22 | 1, 1½, 2 |
When choosing the needle length for an intramuscular injection, the nurse must consider the size of the patient and the amount of fatty tissue. A patient with minimal fatty tissue may need a needle length of 1 inch. For an obese patient, the length of the needle for an intramuscular injection may be 1½ to 2 inches.
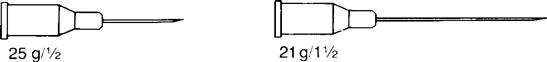
Insulin syringes and pre-filled cartridges have permanently attached needles. With other syringes, needle sizes can be changed. Needle gauge and length are indicated on the syringe package or on the top cover of the syringe. These values appear as gauge/length, such as 21 g/1½ inch. Figure 9-12 shows two types of needle gauge and length.
Research has shown that after an injection, medication remains in the hub of the syringe, where the needle joins the syringe. This volume can be as much as 0.2 mL. There is controversy as to whether air should be added to the syringe before administration to ensure that the total volume is given. The best practice is to follow the institution’s policy.
Angles for Injection
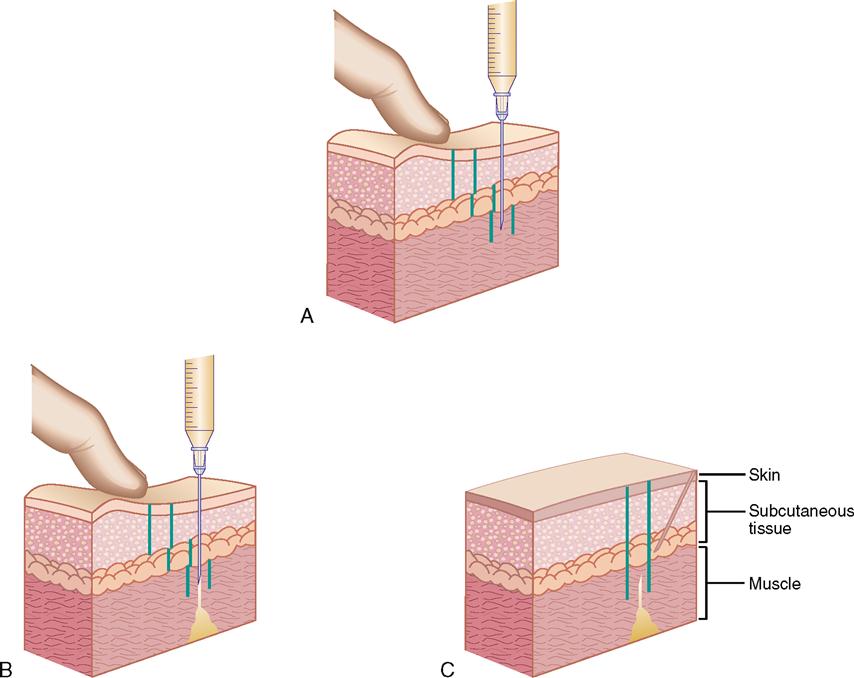
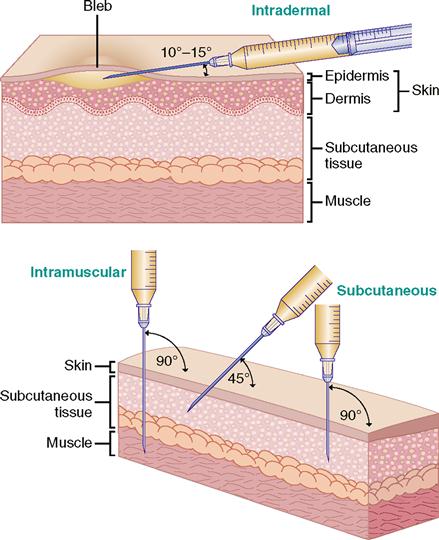
For injections, the needle enters the skin at different angles. Intradermal injections are given at a 10- to 15-degree angle; subcutaneous injections, at a 45- to 90-degree angle; and intramuscular injections, at a 90-degree angle. Figure 9-13 shows the angles for intradermal, subcutaneous, and intramuscular injections.
INTRADERMAL INJECTIONS
Usually, an intradermal injection is used for skin testing. Primary uses are for tuberculin and allergy testing. The tuberculin syringe (25 g/½ inch) holds 1 mL (16 minims) and is calibrated in 0.1 to 0.01 mL.
The inner aspect of the forearm is often used for diagnostic testing because there is less hair in the area and the test results are easily seen. The upper back can also be used as a testing site. The needle is inserted with the bevel upward at a 10- to 15-degree angle. Do not aspirate. Test results are read 48 to 72 hours after the intradermal injection. A reddened or raised area indicates a positive reaction.
SUBCUTANEOUS INJECTIONS
Drugs injected into the subcutaneous (fatty) tissue are absorbed slowly because there are fewer blood vessels in the fatty tissue. The amount of drug solution administered subcutaneously is generally 0.5 to 1 mL at a 45-, 60-, or 90-degree angle. Irritating drug solutions are given intramuscularly because they could cause sloughing of the subcutaneous tissue.
The two types of syringes used for subcutaneous injection are the tuberculin syringe (1 mL), which is calibrated in 0.1 and 0.01 mL, and the 3-mL syringe, which is calibrated in 0.1 mL (Figure 9-14). The needle gauge commonly used is 25 or 26 gauge, and the length is usually ⅜ to ⅝ inch. Insulin is also administered subcutaneously and is discussed later in this chapter.
Calculations for Subcutaneous Injections
Formulas for solving problems of subcutaneous injections are the basic formula, ratio and proportion, fractional equation, and dimensional analysis (see Chapter 6). The following problems are examples of injections that can be given subcutaneously.
EXAMPLES
PROBLEM 1: Order: heparin 5000 units, subQ.
Drug available:

Methods:
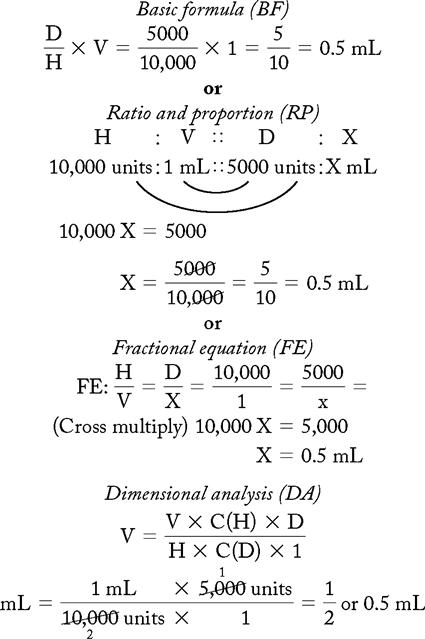
Answer: Heparin 5000 units = 0.5 mL
PROBLEM 2: Order: morphine 10 mg, subQ.
Drug available:

See label with approximate equivalents.
Methods:
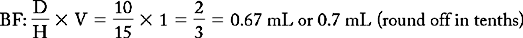
or
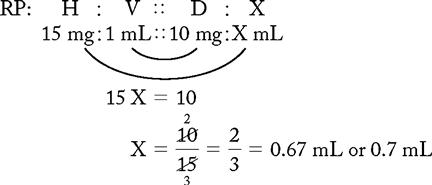
or
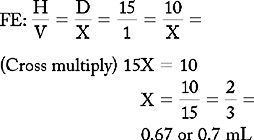
or
DA: no conversion factor
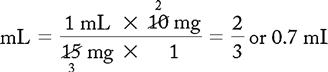
Answer: Morphine 10 mg = 0.67 or 0.7 mL (use a tuberculin syringe or a 3-mL syringe). (Round off in tenths.)
INSULIN INJECTIONS
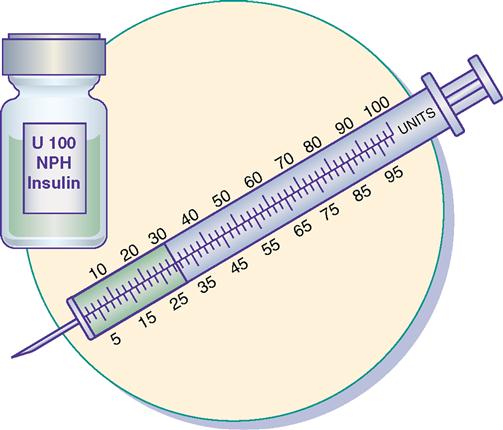
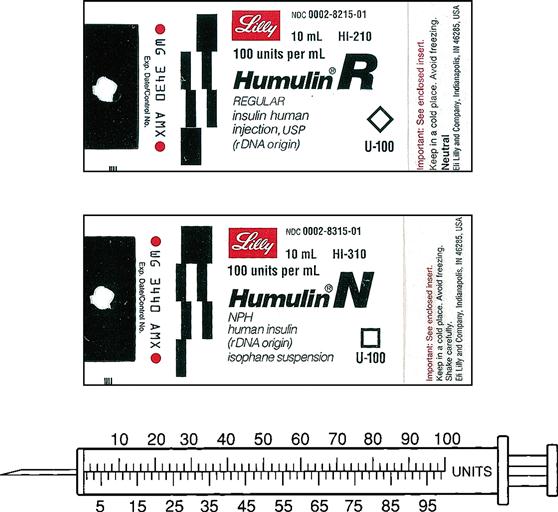
Insulin is prescribed and measured in USP units. Most insulins are manufactured in concentrations of 100 units per milliliter. Insulin should be administered with an insulin syringe, which is calculated to correspond with the units 100 insulin. Insulin bottles and syringes are color-coded. The units 100 insulin bottle and the units 100 insulin syringe are color-coded orange.
The insulin syringe may be marked on one side in even units (10, 20, 30) and on the other side in odd units (5, 15, 25, etc.).
Insulin is easy to prepare and administer as long as the nurse uses the same insulin concentration with the same calibrated insulin syringe, e.g., a units 100 insulin bottle and a units 100 insulin syringe. For example, if the prescribed insulin dosage is 30 units, withdraw 30 units from a bottle of units 100 insulin using a units 100–calibrated insulin syringe.
TYPES OF INSULIN
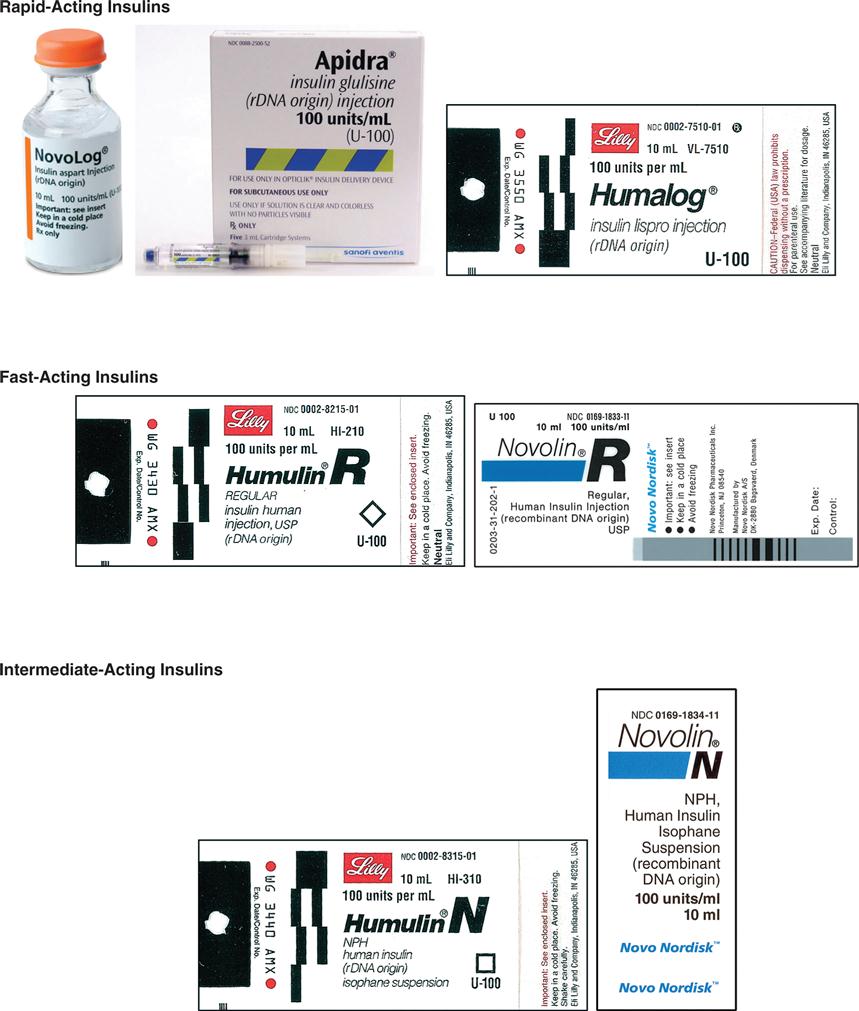
Insulin is categorized as fast-acting, intermediate-acting, long-acting, and commercial premixed insulin. The following drug labels are arranged according to insulin action.
Insulin has been available since 1925, when it was extracted from beef and pork pancreases. In the 1980s, human insulin was synthetically produced and began replacing beef and pork insulin in the United States. Some individuals are allergic to beef insulin, so since 1998, beef insulin has no longer been manufactured. Pork insulin was more frequently prescribed because it has biologic properties similar to those of human insulin. Since December 2005, pork insulin has not been available in the United States. Some patients may be importing it. Now, insulin analogs have been developed and are currently in use.
Insulins have various descriptions, including color, action, source, and manufacturer. They are either clear (regular or crystalline insulin) or cloudy (NPH) because of the substance, protamine, used to prolong the action of insulin in the body. Insulin action is broken down into onset, peak, and duration. Onset is how long it takes the insulin to begin working. Peak is when the insulin is working most effectively, and duration is how long the insulin remains effective. Additionally, insulins are either DNA recombinant or analogs. Since 2005 only human insulin has been available in the United States. Human insulin is DNA recombinant and is manufactured; it does not come from cadavers. Analog insulin is human insulin that has been manipulated to change the action. The three insulin manufacturers are Eli Lilly, NovoNordisk, and Sanofi-Aventis. Humulin and Novolin are examples of brand names of insulins.
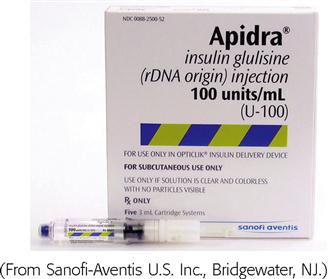
Insulin is categorized as rapid-acting, fast-acting, intermediate-acting, long-acting, and commercial premixed insulin (see Figures 9-15 and 9-16). Insulin is prescribed in units and administered in units. The first rapid-acting insulin, Humalog (lispro insulin), was approved for use in 1996. Lispro and the new rapid-acting insulins, aspart and glulisine, act faster than regular insulin and thus can be administered 5 to 15 minutes before mealtime, whereas regular insulin is given 30 minutes before meals. Rapid-acting insulins can become effective within 5 to 15 minutes of injection and last 3 to 5 hours. Lispro insulin (Humalog) is formed by reversing two amino acids in human regular insulin (Humulin R). Aspart insulin (NovoLog) is an analog of human insulin with a rapid onset. It is structured identically to human insulin expect for one amino acid. Glulisine insulin (Apidra), like aspart insulin, is a synthetic analog of natural human insulin (see Table 9-2 and Figure 9-15). Rapid-acting (Aspart, Apidra, and Humalog) and fast-acting (regular) insulins can be given intravenously as well as subcutaneously. Intermediate-acting and long-acting insulins can ONLY be administered subcutaneously.
TABLE 9-2
| ACTION | |||||||
| Generic (Brand) | Route | Color | Pregnancy Category | Time to Administer | Onset | Peak | Duration (Dose-Related) |
| Rapid-Acting Insulin (Short Duration) | |||||||
| aspart (NovoLog) | A: subQ, IV | Clear | B | 5-15 min before meals | 5-15 min | 1-3 h | 3-5 h |
| glulisine (Apidra) | A: subQ, IV | Clear | B | 5-15 min before meals | 5-15 min | 1-2 h | 3-4.5 h |
| lispro (Humalog) | A: subQ, IV | Clear | B | 5-15 min before meals | 5-15 min | 0.5-2 h | 3-5 h |
| Fast-Acting Insulin (Slower Duration) | |||||||
| regular insulin (Humulin R, Novolin R) | A, C: subQ, IV | Clear | B | 15-30 min before meals | 0.5-1 h | 2-4 h | 6-8 h |
| Intermediate-Acting Insulin | |||||||
| NPH Insulin, Humulin N | A, C: subQ | Cloudy | B | 30 min before meals | 1-2 h | 6-12 h | 12-18 h |
| Long-Acting Insulin | |||||||
| determir (Levemir) | A: subQ | Clear | C | Dinner or bedtime | 1-2 h | 6-8 h | 14-24 h (dose related) |
| glargine (Lantus) | A: subQ | Clear | C | Bedtime | 1.5-2 h | No peak | 24 h |
| COMBINATIONS | |||||||
| Rapid- and Intermediate-Acting Insulin | |||||||
| 70% aspart protamine/30% aspart insulin (NovoLog mix 70/30) | Cloudy | B | 15 min before meals | 15 min | 1-4 h | 12-18 h | |
| 75% lispro protamine/25% lispro insulin (Humalog mix 75/25) | A: subQ | Cloudy | B | 15 min before meals | 15 min-2h | 2-6 h | 14-18 h |
| Fast- and Intermediate-Acting Insulin | |||||||
| 70% NPH/30% regular nsulin (Humulin 70/30, Novolin 70/30) | A: subQ | Cloudy | B | 15 min before meals | 30-60 min | 2-8 h | 10-18 h |
| 50% NPH/50% regular nsulin (Humulin 50/50) | A: subQ | Cloudy | B | 15 min before meals | 15-60 min | 2-6 h | 10-18 h |
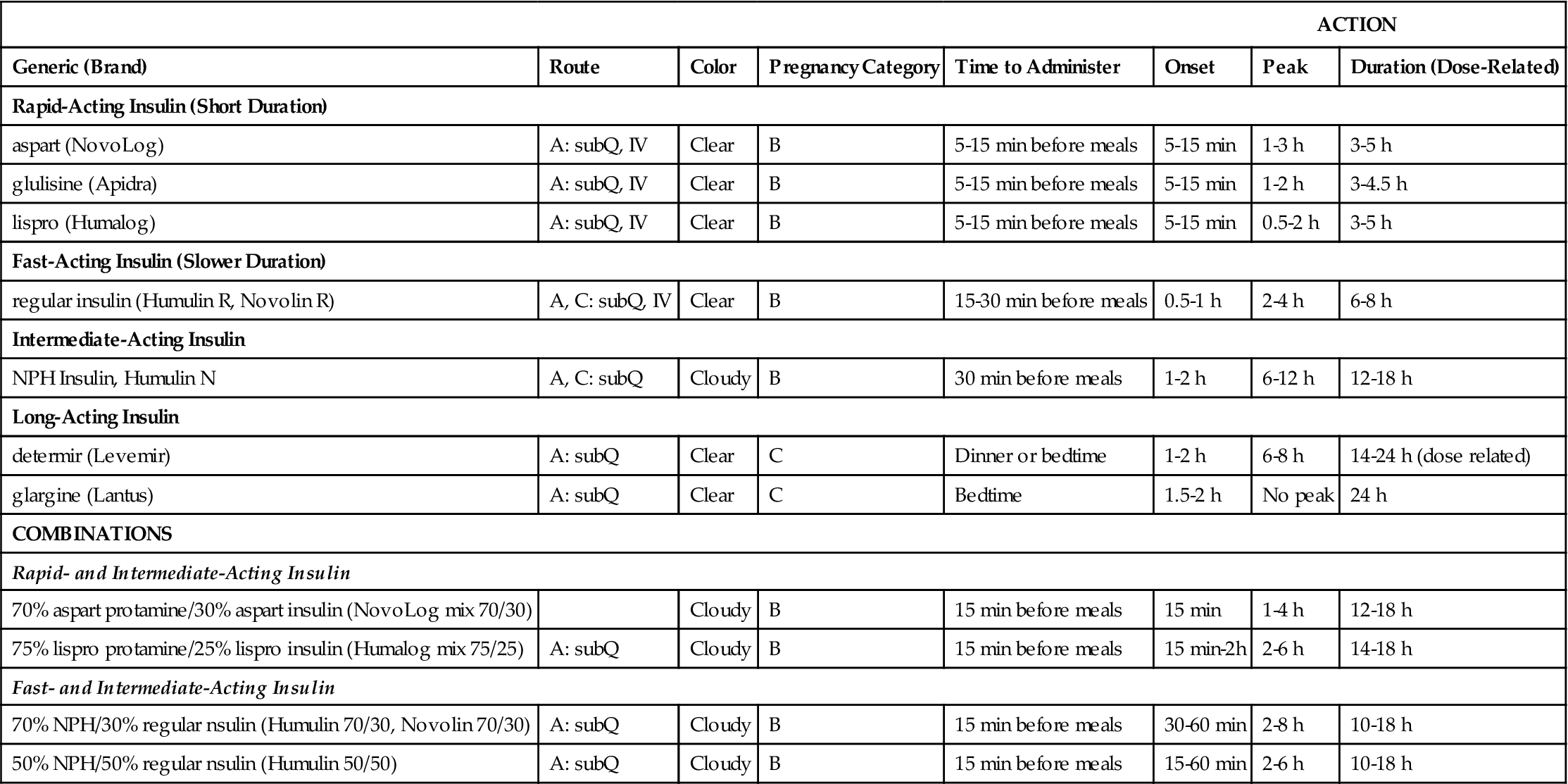
A, adult; Q child; h, hour; min, minute; subQ; subcutaneous; IV, intravenous, <, less than.
CAUTION: Levemir and Lantus should NOT be mixed with other insulins and should NEVER be given intravenously.
Fast-acting insulin (regular insulin) is also clear but takes longer to start working compared with rapid-acting insulins. It is administered 30 minutes before meals and is effective for 6 to 8 hours. If it is given during or after the meal, the patient may experience low blood sugars. Fast-acting insulin is known as regular or R insulin. Humulin R and Novolin R are brand names of fast-acting human insulin.
Intermediate-acting insulin (NPH, Humulin N, Novolin N) is administered 30 minutes before meals (breakfast) and becomes effective in 1 to 2 hours. Its duration of action in the body is 12 to 18 hours. This type of insulin contains protamine, which prolongs the action in the body. It is cloudy because of the protamine added to the regular insulin. It can ONLY be given subcutaneously. Humulin N can be mixed with Humulin R (regular insulin) or rapid-acting insulin in the same syringe.
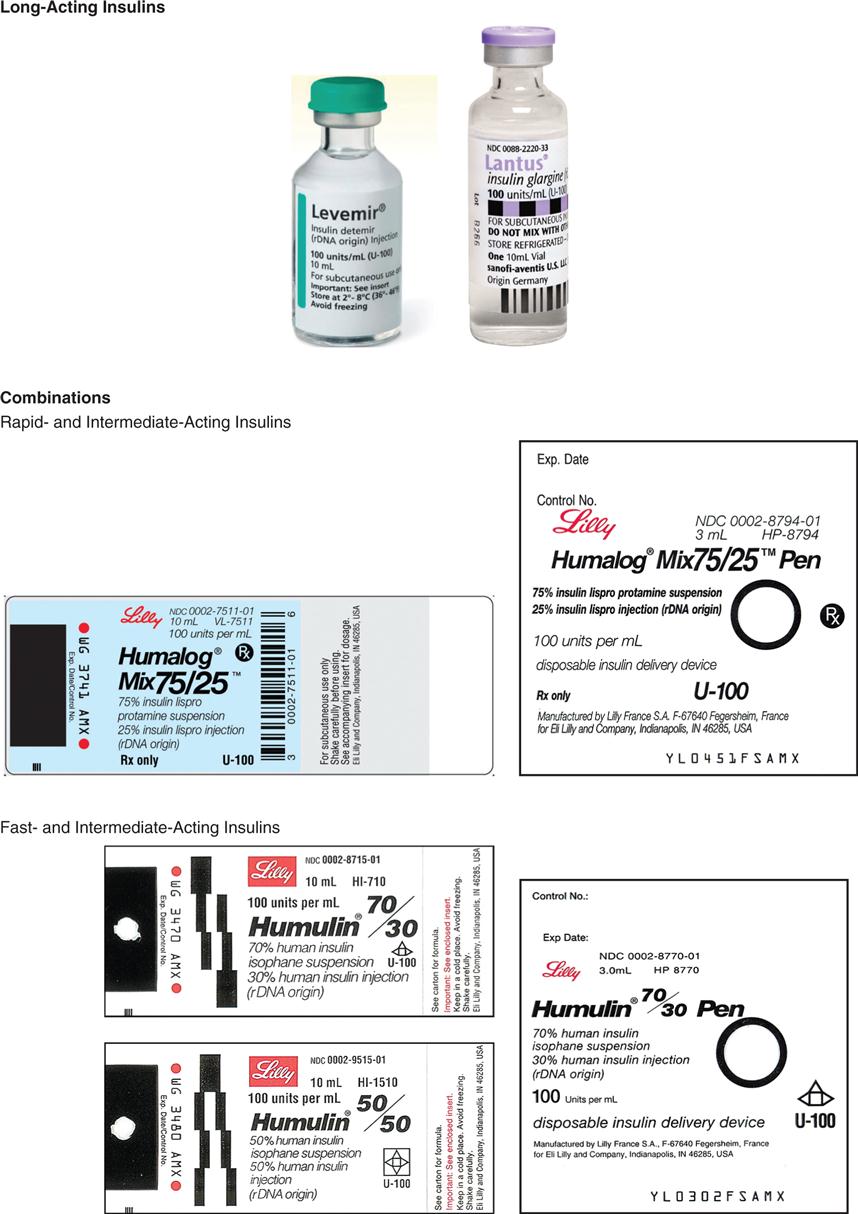
The long-acting insulins are insulin detemir (Levemir), an analog of human insulin, and insulin glargine (Lantus). Lantus is the first long-acting recombinant DNA (rDNA) human insulin for patients with type 1 and 2 diabetes mellitus. Lantus and Levemir are clear, colorless insulins that are to be given ONLY subcutaneously and NOT intravenously. Lantus and Levemir CANNOT be mixed with other insulins or given intravenously. The long-acting insulin acts within 1 to 2 hours and last in the body for 18 to 24 hours. The Levemir vial is tall and has a green top. The Lantus vial is taller and narrower than the other types of insulin. It has a purple top and purple print on the label. Levemir is usually administered in the evening or at bedtime; however, it can be administered once or twice a day subcutaneously. Lantus is usually administered at bedtime, thus, the incidence of noctural hypoglycemia is not common. Some patients report more pain at the injection site with long-acting insulins than with Humulin N (NPH).
The use of commercially premixed combination insulins has become popular for patients with diabetes mellitus who mix fast-acting and intermediate-acting insulins. Examples are two groups: the rapid- and intermediate-acting insulin and the fast- and intermediate-acting insulins. The two rapid- and intermediate-acting insulins are Novolog mix 70/30 and Humalog mix 75/25. The fast- and intermediate-acting insulins are Humulin 70/30, Novolin 70/30, and Humulin 50/50 (see Table 9-2). They are available in vials or pens that resembles a fountain pen. Some patients need less than 30% Humulin R and more Humulin N, so these combinations of insulins cannot be used. They must mix their insulins according to the prescribed units of insulin.
Insulin is administered at a 45-, 60-, or 90-degree angle into the subcutaneous tissue. The angle for administering insulin depends on the amount of fatty tissue in the patient. For a very thin person, a 45-degree angle is suggested. A 90-degree angle should be used for obese or average-sized persons. When a 90-degree angle is used, the skin should be pinched upward so the insulin is deposited into the fatty tissue.
MIXING INSULINS
Regular insulin is frequently mixed with insulins containing protamine, such as Humulin N.
REMEMBER: Insulin is prescribed in units and administered in units.
EXAMPLE
Problem and method for mixing insulin.
PROBLEM: Order: Humulin R insulin units 10 and Humulin N insulin units 40, subQ.
Drug available: Humulin R insulin units 100 and Humulin N insulin units 100, both in multidose vials. The insulin syringe is marked units 100.
2. Draw up 40 units of air* and inject into the Humulin N insulin bottle. Do not allow the needle to come into contact with the Humulin N insulin solution. Withdraw the needle.
3. Draw up 10 units of air and inject into the Humulin R insulin bottle.
4. Withdraw 10 units of Humulin R insulin. Humulin R insulin is withdrawn before Humulin N insulin.
5. Withdraw 40 units of Humulin N insulin.
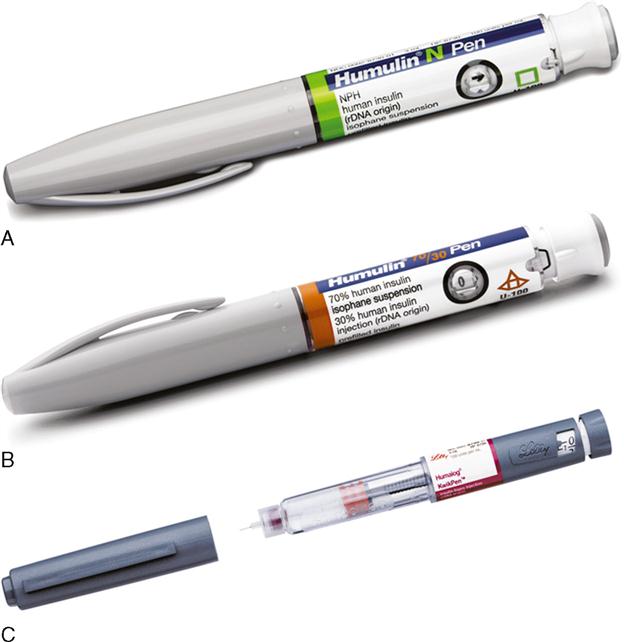
Insulin Pen Devices
There are two types of insulin pen devices: pre-filled and reusable. Both types require insulin pen needles to dispense the insulin.
Pre-filled insulin pen devices are filled with 300 units or 3 mL of units-100 insulin. Before each insulin dose, a small disposable needle is placed on the end of the insulin pen device and then the insulin dose is dialed in. As the dose is dialed in, the plunger comes out. After the dose is dialed, the needle is placed subcutaneously and the plunger pushed down. After the insulin is delivered, the dose indicator returns to zero and the needle is removed from the skin. The needle is removed. A new needle is placed on the prefilled pen before each injection. The pre-filled pen device is reused for multiple injections until all the insulin is dispensed. Once the insulin is completely dispensed, the pen is thrown away (Figure 9-17).
Reusable insulin pen devices are filled with disposable insulin cartridges. The cartridges are filled with 150 units (1.5 mL) or 300 units (3 mL) of units-100 insulin. The cartridge is placed in the pen device. Before each insulin dose, a small disposable needle is placed on the end of the insulin pen device and then the insulin dose is dialed in. As the dose is dialed in, the plunger comes out. After the dose is dialed, the needle is placed subcutaneously and the plunger pushed down. After the insulin is delivered, the dose indicator returns to zero and the needle is removed from the skin. The needle is removed from the pen device. The pre-filled pen device is reused for multiple injections until all the insulin is dispensed from the cartridge. Once the cartridge is empty, the cartridge is thrown away and a new cartridge placed in the reusable pen device.
INSULIN PUMPS
There are two types of insulin pumps—the implantable and the external (portable). The implantable insulin pump is surgically implanted in the abdomen and delivers a basal insulin infusion and bolus doses with meals either intravenously or intraperitoneally. With implantable insulin pumps, there are fewer hypoglycemic reactions, and blood glucose levels are mostly controlled.
External (portable) insulin pumps, also called continuous subcutaneous insulin infusion or CSII, have been available since 1983. CSII mimics the body’s normal delivery of insulin. The external insulin pump keeps blood glucose (sugar) levels as close to normal as possible. The continuous delivery of insulin is called the basal rate and the larger pre-meal doses are called bolus doses. The insulin delivery setting is programmed by the diabetes expert and adjusted by the patient. Before the patient eats, the pump is programmed to dispense a large dose through the catheter. The patient then (1) programs infuse insulin at a basal rate of units per hour (but which can be adjusted), (2) delivers bolus infusions to cover meals (the patient pushes a button to deliver a bolus dose at meals), (3) changes delivery rates at specific times of the day (e.g., from 3 AM to 9 AM) to avoid early-morning hyperglycemia, and (4) overrides the set basal rate to allow for unexpected changes in activity such as early-morning exercise.
Most insulin pump systems consist of the insulin pump, an insulin reservoir, plastic tubing, and insertion set. The insulin reservoir holds 150 to 300 units of rapid or fast-acting insulin, which is held in the insulin pump. The plastic tubing is attached to a metal or plastic needle and placed subcutaneously by the patient. The needle can be inserted into the abdomen, upper thigh, or upper arm. Only regular insulin is used because protamine insulin, such as Humulin N, can cause unpredictable blood glucose levels. The pump can deliver small amounts of insulin such as 0.1 or 0.2 units much more accurately than a traditional insulin syringe. Again, these pumps used a remote control to program the basal rates and bolus doses. The patient usually changes the insertion site every 3 days.
A glucose sensor device is available to check the fluid glucose level. The sensor is separate from the insulin pump and is attached to the body surface area. Radio-like wave sounds are transmitted to the pump, which records the glucose level on the pump every 5 minutes. An alarm warns of low or high glucose levels.
The use of the insulin pump helps to decrease the risk of severe hypoglycemic reactions and maintains glucose control. However, glucose levels should still be monitored at least daily with or without an insulin pump. The person with type 1 diabetes mellitus has the greatest benefit from use of an insulin pump. This method should reduce the number of long-term diabetic complications compared with the use of multiple injections of regular and modified types of insulins. Figure 9-18 shows an example of an insulin pump.
INTRAMUSCULAR INJECTIONS
The IM injection is a common method of administering injectable drugs. The muscle has many blood vessels (more than fatty tissue), so medications given by IM injection are absorbed more rapidly than those given by subQ injection. The volume of solution for an IM injection is 0.5 to 3.0 mL, with the average being 1 to 2 mL. A volume of drug solution greater than 3 mL causes increased muscle tissue displacement and possible tissue damage. Occasionally, 5 mL of certain drugs, such as magnesium sulfate, may be injected into a large muscle, such as the dorsogluteal. Dosages greater than 3 mL are usually divided and are given at two different sites.
Needle gauges for IM injections containing thick solutions are 19 gauge and 20 gauge, and for thin solutions, 20 gauge to 21 gauge. IM injections are administered at a 90-degree angle. The needle length depends on the amount of adipose (fat) and muscle tissue; the average needle length is 1½ inches.
The Z-track injection technique delivers medication intramuscularly in a method that prevents the drug from leaking back into the subcutaneous tissue (Figure 9-19). This method is ordered for medications that could cause irritation to the subcutaneous tissue or discoloration to the skin. When preparing the medication, a needle change is made after the drug has been pulled up into the syringe and before it is injected into the patient. The large gluteal muscle is frequently used.
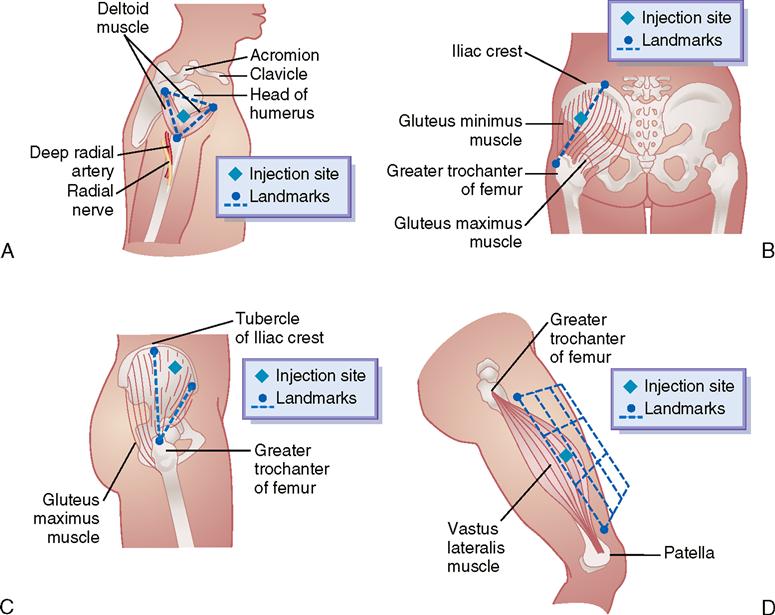
Common sites for IM injections are the deltoid, dorsogluteal, ventrogluteal, and vastus lateralis muscles. Figure 9-20 displays the sites for each muscle used with IM injection. Table 9-3 gives the volume for drug administration, common needle size, patient’s position, and angle of injection for the four IM injection sites.
TABLE 9-3
Intramuscular Injection Sites in the Adult
| Deltoid | Dorsogluteal | Ventrogluteal | Vastus Lateralis | |
| Volume for drug administration | ||||
| Common needle size | 23-25 gauge; 5/8-1½ inches | 18-23 gauge; l¼–3 inches | 20-23 gauge; 1¼-2½ inches | 20-23 gauge; 1¼–1½ inches |
| Patient’s position | Sitting; supine; prone | Prone | Supine; lateral | Sitting (dorsiflex foot); supine |
| Angle of injection | 90-degree angle, angled slightly toward the acromion | 90-degree angle to flat surface; upper outer quadrant of the buttock or outer aspect of line from the posterior iliac crest to the greater trochanter of the femur | 80-degree—90-degree angle; angle the needle slightly to-ward the iliac crest | Stay updated, free articles. Join our Telegram channel
Full access? Get Clinical Tree
 Get Clinical Tree app for offline access
Get Clinical Tree app for offline access

|

 I NEEDLES
I NEEDLES II SUBCUTANEOUS INJECTIONS
II SUBCUTANEOUS INJECTIONS




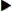 III INSULIN
III INSULIN
 IV INSULIN PEN DEVICES
IV INSULIN PEN DEVICES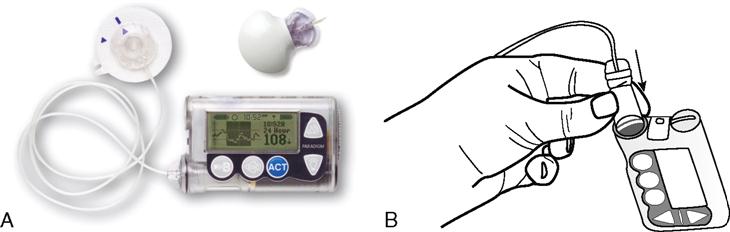
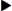 V INSULIN PUMP
V INSULIN PUMP