TOXOCARA
![]() TOXOCARA CANIS: PARASITOLOGY AND LIFE CYCLE (FIGURE 55–1)
TOXOCARA CANIS: PARASITOLOGY AND LIFE CYCLE (FIGURE 55–1)
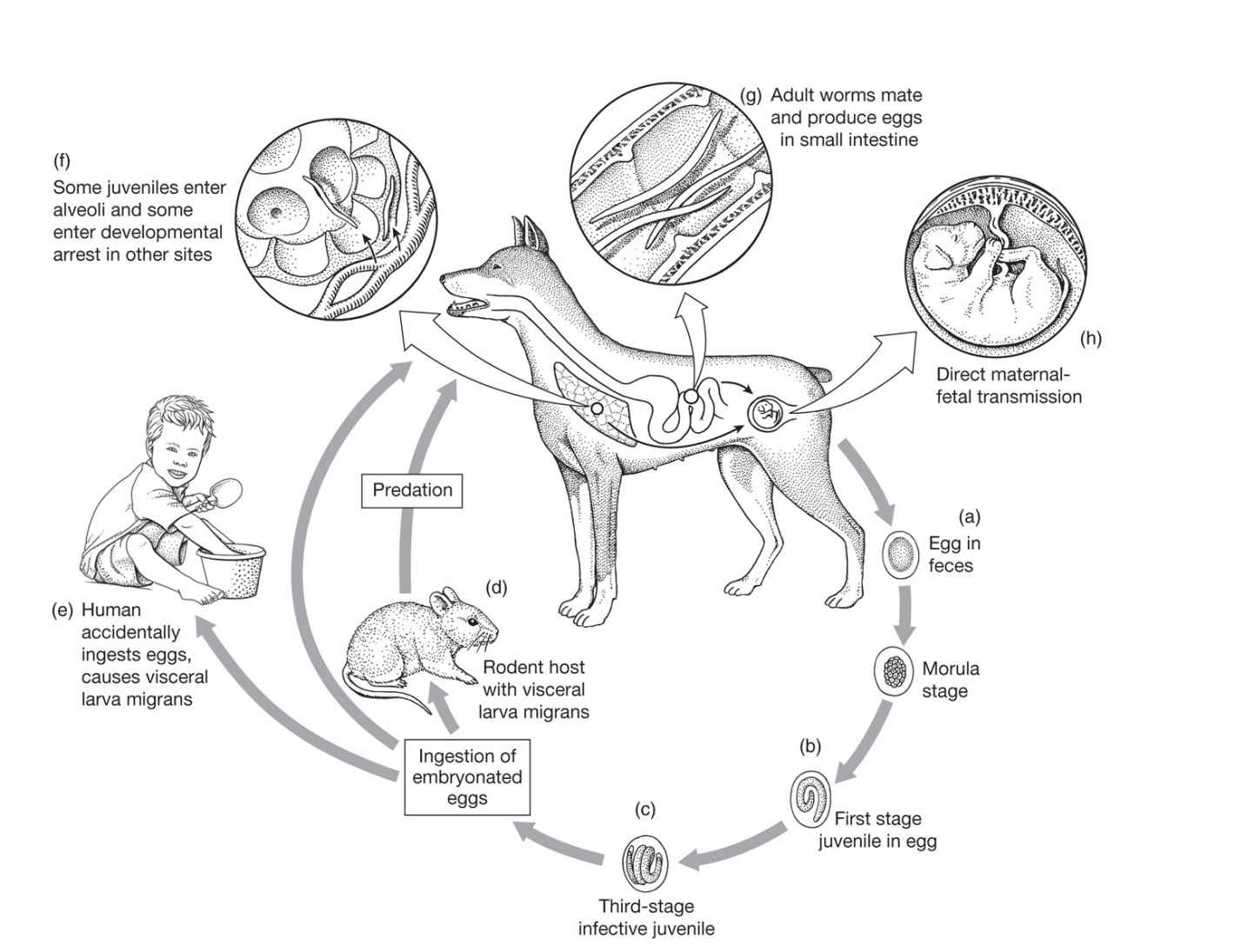
FIGURE 55–1. Life cycle of Toxocara canis. (Reproduced with permission from Roberts RL, Janovy J, Nadler S: Foundations of Parasitology, 9th edition. McGraw-Hill, 2013.)
Toxocara canis is a large, intestinal ascarid of canines, including dogs, foxes, and wolves. Occasionally, a related organism found in cats (T cati) can behave in a similar fashion. Each female worm discharges approximately 200 000 thick-shelled eggs daily into the fecal stream. After reaching the soil, these eggs embryonate for a minimum of 2 to 3 weeks. Thereafter, the eggs are infectious to canines, humans, and other mammals. The eggs may remain infectious in the soil for months to years. When ingested by a puppy, the larvae exit from the eggshell, penetrate the intestinal mucosa, and migrate through the liver and the right side of the heart to the lung. Here, like the offspring of Ascaris lumbricoides, they burst into the alveolar airspaces and are coughed up and swallowed; thereafter, they mature in the small bowel. However, in fully grown dogs, the life cycle is different: Most of the migrating larvae pass through the pulmonary capillaries and reach the systemic circulation. These larvae eventually are filtered out and encyst in the dog’s tissues, where they lie dormant for months or longer. Hormonal changes and/or diminished immunity in the pregnant bitch stimulate the larvae to resume development, migrate across the placenta, and infect the unborn pups. Larvae may also pass to the newborn puppies in their mother’s milk. Approximately 4 weeks after birth, both the puppies and the lactating mother begin to pass large numbers of eggs in their stools, shed by the adult worms that inhabit their intestinal tract. These eggs embryonate in the soil for 2 to 3 weeks before becoming infectious. The mother may then be superinfected by ingesting the newly eggs from the soil or eating visceral cysts in an intermediate host such as a rodent.
Cycle in canines resembles ascariasis in humans, but with tissue cysts
Transplacentally infected puppies and lactating bitches excrete numerous ova
Eggs embryonate 2 to 3 weeks in soil
When humans ingest infectious eggs, the liberated larvae are small enough to pass through the pulmonary capillaries and reach the systemic circulation. Only rarely do larvae break into the alveoli, get coughed up and swallowed to reach the intestine to mature into adults. Instead, larvae in the systemic circulation continue to grow there. When their size exceeds the diameter of the vessel through which they are passing, they penetrate its wall and enter the tissue. The larvae induce a TH2-type CD4+ response characterized by eosinophilia and IgE production.
Transmission to humans by ingestion of ova
Larvae invade tissues and encyst instead of returning to GI tract via lung
![]() TOXOCARIASIS
TOXOCARIASIS
EPIDEMIOLOGY
Toxocara canis is a cosmopolitan parasite. The infection rate in the 50 million dogs inhabiting the United States is very high; over 80% of puppies and 20% of older animals are parasitized. “Man’s best friend” deposits more than 3500 tons of feces daily in the streets, yards, and parks of America, and there is a real health risk. In some areas, between 10% and 30% of soil samples taken from public parks have contained viable Toxocara eggs. Moreover, serologic surveys of humans indicate that approximately 4% to 20% of the population has ingested these eggs at some time. The incidence of infection appears to be higher in the Southeastern United States; presumably the warm, humid climate prolongs survival of the eggs, thereby increasing exposure. Indeed, seroprevalence rates of more than 50% have been noted in some developing nations. Puppies in the home increase the risk of infection. Clinical manifestations occur predominantly among children 1 to 6 years of age; many have a history of geophagia, suggesting that disease transmission results from direct ingestion of eggs in the soil. Most infections are subclinical, but the incidence of overt disease is likely underreported.
Soil contaminated with ova deposited by domestic dogs
Children are more often infected
Infection more common than disease, but disease underreported
 TOXOCARIASIS: CLINICAL ASPECTS
TOXOCARIASIS: CLINICAL ASPECTS
MANIFESTATIONS
The larvae of Toxocara that reach the systemic circulation may invade any tissue of the human body, where they can induce necrosis, bleeding, eosinophilic granulomas, and subsequent fibrosis. The liver, lungs, heart, skeletal muscle, brain, and eye are involved most frequently. The severity of clinical manifestations is related to the number and location of these lesions and the degree to which the host has become sensitized to larval antigens. Children with more intense infection may have fever and an enlarged, tender liver. Those who are seriously ill may develop a skin rash, an enlarged spleen, asthma, recurrent pulmonary infiltrates, abdominal pain, sleep and behavioral changes, focal neurologic defects, and seizures. This illness, called “visceral larva migrans,” often persists for weeks to months. Death may result from respiratory failure, cardiac arrhythmia, or brain damage. In older children and adults, these systemic manifestations are uncommon, although eye invasion by larvae (“ocular larva migrans”) is more common. Typically, unilateral strabismus (squint) or decreased visual acuity causes the patient to consult an ophthalmologist. Examination reveals granulomatous endophthalmitis, which is usually a reaction to a larva that is already dead; it is sometimes mistaken for malignant retinoblastoma, and unnecessary enucleations have been performed.
Any tissue can be invaded by larvae
Organ invasion causes hypersensitivity
Ocular invasion produces granulomatous endophthalmitis
DIAGNOSIS
Stool examination for eggs is not helpful because the parasite seldom reaches adulthood in humans. Definitive diagnosis requires demonstration of the larva in a liver biopsy specimen or at autopsy. A presumptive diagnosis may be made based on the clinical picture: Eosinophilic leukocytosis, elevated serum levels of IgE, and elevated antibody titers to blood group antigens, particularly the group A antigen. An enzyme immunoassay (EIA) using larval antigens has been developed, providing clinicians with a reasonably sensitive (75%) and specific (90%) serologic test. A western blot procedure is somewhat more sensitive but is not widely available. Unfortunately, many patients with related ocular infections remain seronegative; some demonstrate elevated antibody titers within the ocular fluids.
Tissue biopsy required for detection
Serodiagnosis using EIA imperfectly reliable
TREATMENT AND PREVENTION
Reduction of the exuberant host immune response is the main goal of treatment. Corticosteroid treatment may be lifesaving if the patient has serious pulmonary, myocardial, or central nervous system (CNS) involvement. Anthelmintic therapy with albendazole is often administered, although the efficacy of this drug remains uncertain. Prevention requires control of indiscriminate defecation by dogs and repeated deworming of household pets. Deworming must begin when the animal is 3 weeks of age and should be repeated every 3 months during the first year of life and twice a year thereafter.
Corticosteroids in serious disease; antihelminthic treatment less certain
Disposal of pet feces, deworming, avoidance of geophagia
BAYLISASCARIS
Another nematode that shares clinical and epidemiologic similarities with Toxocara has been increasingly recognized. Baylisascaris procyonis (raccoon roundworm) has predominantly affected children playing in wooded areas that are frequented by raccoons. Raccoon “latrines” may teem with infective eggs, which, when accidentally ingested, may cause a disease that mimics toxocariasis. Unfortunately, this organism has a particular predilection for neural and eye tissue, and can lead to devastating eosinophilic meningoencephalitis and retinitis. The diagnostic and therapeutic approaches are similar to those for Toxocara, but the clinical outcome may be fatal, especially when therapy is delayed.
Raccoon roundworm mimics Toxocara, but can especially be lethal
TRICHINELLA
![]() TRICHINELLA SPIRALIS: PARASITOLOGY AND LIFE CYCLE
TRICHINELLA SPIRALIS: PARASITOLOGY AND LIFE CYCLE
Adult Trichinella live in the duodenal and jejunal mucosa of flesh-eating animals throughout the world, particularly swine, rodents, bears, canines, felines, and marine mammals. Originally thought to be members of a single species, it is now clear that arctic, temperate, and tropical strains of Trichinella demonstrate significant epidemiologic and biologic differences, and they have been reclassified into eight distinct species. Only two species, T spiralis and the arctic species T nativa, display a high level of pathogenicity for humans. This discussion focuses on the former, while highlighting the unique epidemiologic and clinical characteristics of the latter.
Intestinal parasites of many flesh-eating mammals
Within the host intestinal tissue, the tiny (1.5 mm) male copulates with his larger (3.5 mm) mate and, apparently spent by the effort, dies. Within 1 week, the inseminated female begins to discharge offspring. Unlike those of most nematodes, these progeny undergo intrauterine embryonation and are released as second-stage larvae. The birthing continues for the next 4 to 16 weeks, resulting in the generation of some 1500 larvae, each measuring 6 by 100 μm.
From their submucosal position, the larvae find their way into the vascular system and pass from the right side of the heart through the pulmonary capillary bed to the systemic circulation, where they are distributed throughout the body. Larvae penetrating tissue other than skeletal muscle disintegrate and die. Those finding their way to striated muscle continue to grow, molt, and gradually encapsulate over a period of several weeks. Calcification of the cyst wall begins 6 to 18 months later, but the contained larvae may remain viable for 5 to 10 years (Figure 55–2). The muscles invaded most frequently are the extraocular muscles of the eye, the tongue, the deltoid, pectoral, and intercostal muscles, the diaphragm, and the gastrocnemius. If a second animal feeds on the infected flesh of the original host, the encysted larvae are freed by gastric digestion, penetrate the columnar epithelium of the intestine, and mature just above the lamina propria. This cycle is summarized in Figure 55–3.
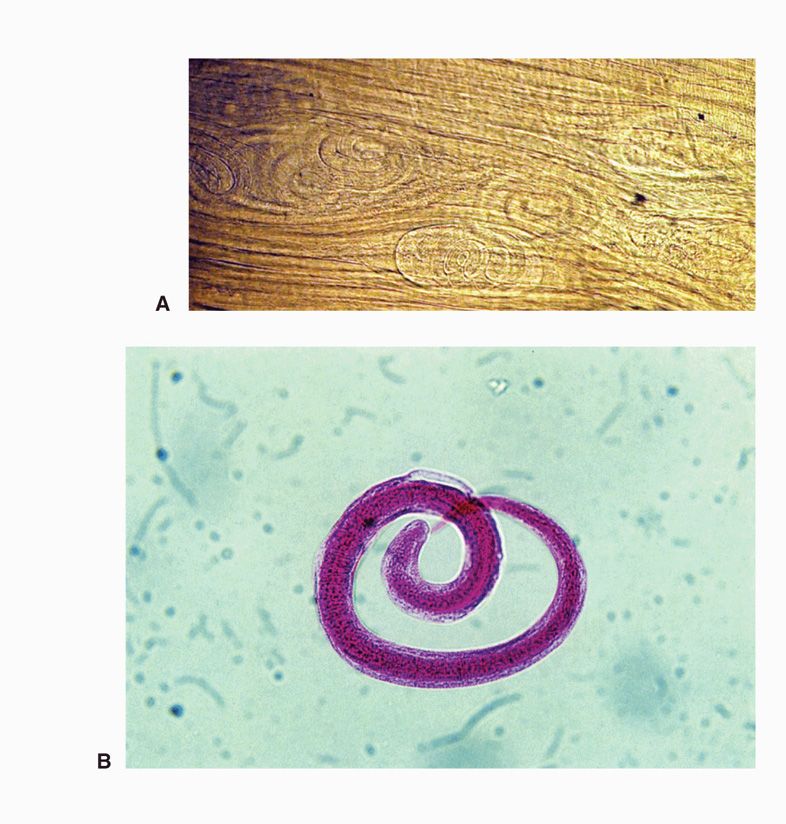
FIGURE 55–2. Trichinella spiralis larvae. A. Coiled larvae in a “squash prep” of deltoid muscle biopsy, in which a small sliver of muscle is squashed under the cover slip and examined without further fixation (image by Paul Pottinger MD). B. Coiled larva from a muscle digest. (Reproduced with permission from Connor DH, Chandler FW, Schwartz DQ, et al: Pathology of Infectious Diseases. Stamford CT: Appleton & Lange, 1997.)
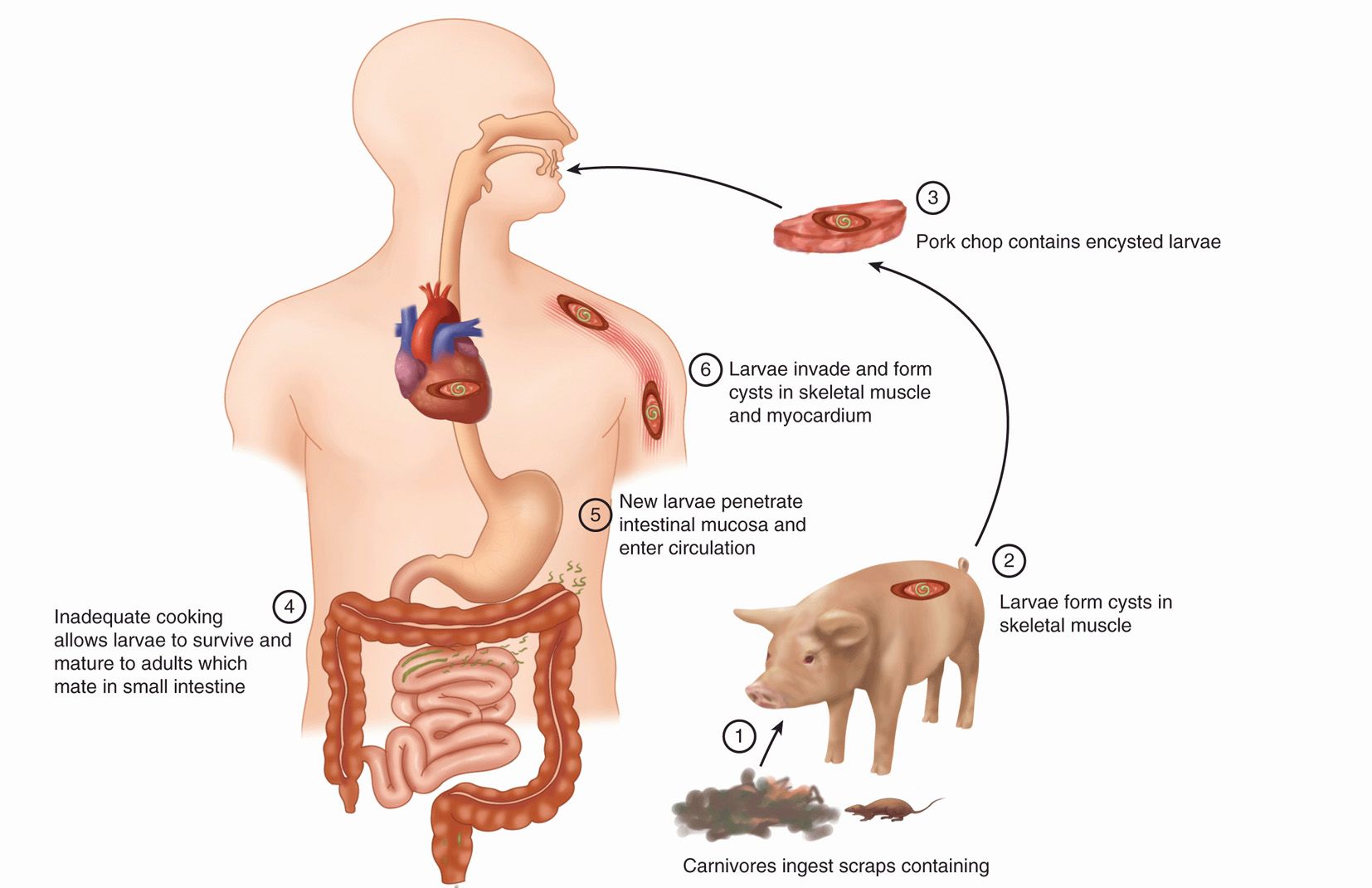
FIGURE 55–3. Trichinosis. Trichinella spiralis larvae ingested by pig (1) eventually end up as human cysts (6).
Larvae reach striated muscle and encapsulate but are still viable
Eating infected flesh spreads the disease
![]() TRICHINOSIS
TRICHINOSIS
EPIDEMIOLOGY
Trichinosis, also called trichinellosis, is widespread in carnivores worldwide. Among domestic animals, swine are most frequently involved. They acquire the infection by eating dead rats or garbage containing cyst-laden scraps of uncooked meat. Human infection, in turn, results largely from the consumption of improperly prepared pork products. In the United States, agricultural regulations have greatly reduced the incidence of trichinosis, and most pig-associated outbreaks have been traced to pork sausage prepared in the home or in small, unlicensed butcheries. Clusters have also followed feasts on wild pig in California and Hawaii. At present, however, the majority of human cases in the United States, particularly those in Alaska and other western states, have been attributed to consumption of wild animal meat, especially bear meat. Outbreaks among Alaskan and Canadian Inuit populations have followed the ingestion of raw T nativa-infected walrus meat. Several recent outbreaks in Europe have involved horse meat or wild boar flesh. In other areas of the world, infection is commonly acquired from wild animals (“sylvatic sources”), including wild boar, bush pigs, and warthogs.
Swine infected by eating rats or meat in garbage
Human infection most often from undercooked pork or wild animals
Human infections occur worldwide. In the United States, the prevalence of cysts found in the diaphragms of patients at autopsy has declined substantially. This decline has been attributed to decreased consumption of pork and pork products; federal guidelines for the commercial preparation of such foodstuffs; the widespread practice of freezing pork, which kills all but arctic strains of T nativa; and legislation requiring the thorough cooking of any meat scraps to be used as hog feed. Nevertheless, it is estimated that many Americans carry live Trichinella in their musculature and that more acquire it annually. Fortunately, the overwhelming majority has a small number of larvae and are asymptomatic. Only about 12 clinically recognized cases are reported annually to federal officials.
Prevalence declining as a result of meat inspection and cooking and freezing pork
Human infections are usually subclinical
PATHOGENESIS AND IMMUNITY
The pathologic lesions of trichinosis are related almost exclusively to the presence of larvae in the striated muscle, heart, and CNS. Invaded muscle cells enlarge, lose their cross-striations, and undergo a basophilic degeneration. Intense inflammation surrounds the involved area, consisting of neutrophils, lymphocytes, and eosinophils. With the development of specific IgG and IgM antibodies, eosinophil-mediated destruction of circulating larvae begins, production of new larvae is slowed, and the expulsion of adult worms is hastened. A vasculitis demonstrated in some patients has been attributed to deposition of circulating immune complexes in the walls of the vessels.
Larvae in striated muscle, heart, and CNS
Acute inflammation with eosinophil-mediated destruction of larvae
![]() TRICHINOSIS: CLINICAL ASPECTS
TRICHINOSIS: CLINICAL ASPECTS
MANIFESTATIONS
One or two days after the host has ingested tainted meat, the newly matured adults penetrate the intestinal mucosa, producing nausea, abdominal pain, and diarrhea. In mild infections, these symptoms may be overlooked, except in a careful retrospective analysis; in more serious infections, they may persist for several days and render the patient prostrate. Diarrhea persisting for a period of weeks has been characteristic of T nativa outbreaks after ingestion of walrus meat by the Inuit population of northern Canada. Larval invasion of striated muscle begins approximately 1 week later and initiates the more characteristic phase of the disease, which may last for about 6 weeks. Patients in whom 10 or fewer larvae are deposited per gram of tissue are usually asymptomatic; those with 100 or more generally develop significant disease; and those with 1000 to 5000 have a very stormy course that occasionally ends in death. Fever, muscle pain, muscle tenderness, and weakness are the most prominent manifestations of trichinosis. Patients may also display eyelid swelling, a maculopapular skin rash, and small hemorrhages beneath the conjunctiva of the eye and the nails of the digits. Hemoptysis and pulmonary consolidation are common in severe infections. If there is myocardial involvement, electrocardiographic abnormalities, tachycardia, or congestive heart failure may be seen. Central nervous system invasion is marked by encephalitis, meningitis, and polyneuritis. Delirium, psychosis, paresis, and coma can follow.
Initial abdominal pain and diarrhea as adults penetrate
Symptoms depend on number and extent of larval muscle invasion
Severe complications include hemoptysis and heart failure
DIAGNOSIS
Trichinosis presents in a clinically protean fashion, which can delay diagnosis and impact clinical outcomes. The most consistent laboratory abnormality is an eosinophilic leukocytosis during the second week of illness, which persists for the remainder of the clinical course. Eosinophils typically range from 15% to 50% of the white cell count, and in some patients, this may induce extensive damage to the cardiac endothelium. In severe or terminal cases, the eosinophilia may disappear altogether. Serum levels of IgE and muscle enzymes are elevated in most clinically ill patients.
Eosinophilia up to 50% starting in second week
There are a number of valuable serologic tests, including indirect fluorescent antibody, bentonite flocculation, and enzyme-linked immunosorbent assay. Significant antibody titers are generally absent before the third week of illness, but may then persist indefinitely.
Antibody usually appears after 2 weeks and then persists
Biopsy of the deltoid or gastrocnemius muscles during the third week of illness often reveals encysted larvae (Figure 55–2B).
Muscle biopsy reveals larvae
TREATMENT
Patients with severe edema, pulmonary manifestations, myocardial involvement, or CNS disease are treated with corticosteroids. The value of specific anthelmintic therapy remains controversial. The mortality rate of symptomatic patients is 1%, rising to 10% if the CNS is involved. Mebendazole and albendazole halt the production of new larvae, but in severe infection, the destruction of tissue larvae may provoke a hazardous hypersensitivity response in the host. This may be moderated with corticosteroids.
Corticosteroids used in severe cases
Antihelminthic therapy used with caution
PREVENTION
Control of trichinosis requires adherence to feeding regulations for pigs, and limiting contact between domestic pigs and wild animals, particularly rodents, who might be carrying Trichinella larvae in their tissues. Domestically, care should be taken to cook pork to an internal temperature of at least 76.6°C, or freeze it at —15°C for 3 weeks before cooking, or thoroughly smoke it before it is ingested. Trichinella nativa in the flesh of arctic animals may survive freezing for a year or more. All strains may survive apparently adequate cooking in microwave ovens due to the variability in the internal temperatures achieved.
Primary prevention involves thorough cooking
CUTANEOUS LARVA MIGRANS
Cutaneous larva migrans, or “creeping eruption,” is an infection of the skin caused by the larvae of a number of animal and human parasites, most commonly the dog and cat hookworm A braziliense. Eggs discharged in the feces of infected animals onto warm, moist, sandy soil then develop into filariform larvae capable of penetrating mammalian skin on contact, just as with human hookworm infection. These parasites are common in tropical areas worldwide; in the United States, parasite transmission is particularly common in the beach areas of the southern Atlantic and Gulf states.
Caused usually by larvae of dog and cat hookworms
Filariform larvae penetrate and migrate in human skin
However, these species are not well adapted to human hosts, and larvae rarely make it across the lung to reach the human intestines or develop further within them. Rather, they may migrate within the skin for a period of weeks to months. Clinically, the patient notes a pruritic, raised, red, irregularly linear lesion 10 to 20 cm long. Skin excoriation from scratching enhances the likelihood of secondary bacterial infection. Half of infected patients develop Lôffler syndrome of transient, migratory pulmonary infiltrations associated with peripheral eosinophilia. The syndrome most probably reflects pulmonary migration of larvae. Larvae are rarely found in either sputum or skin biopsies, and the diagnosis must be established on clinical grounds (Figure 55–4).
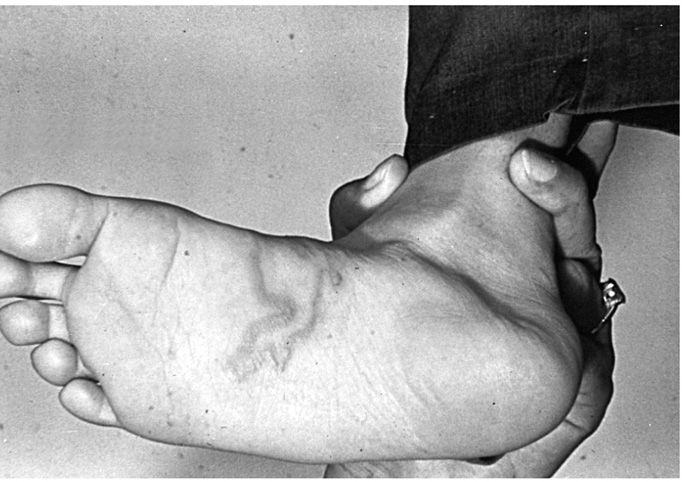
FIGURE 55–4. Creeping eruption caused by infection with Ancylostoma braziliense larva. (Reproduced with permission from Roberts RL, Janovy J, Nadler S: Foundations of Parasitology, 9th edition. McGraw-Hill, 2013.)
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree