HEPATITIS A
 VIROLOGY
VIROLOGY
Hepatitis A virus (HAV) belongs to the Picornaviridae (picornaviruses) family and Hepato-virus genus. It is an unenveloped (naked capsid), single-stranded, positive-sense RNA virus with a cubic (icosahedral) symmetry and a diameter of 27 nm (Figure 13–1). The genome of HAV is a 7.4 kb positive-sense, single-stranded RNA bound to a protein called VPg, and each capsid unit comprises four proteins, VP1, -2, -3, and -4, which cover the genome and form a naked capsid icosahedral virion. VP1 is the spike of HAV that binds to the receptor on the host cells. There is only one serotype of HAV. This virus possesses several characteristics of enteroviruses; for example, it resists inactivation and is stable at –20°C with low pH. The virus has been successfully cultivated in primary marmoset liver cell cultures and in fetal rhesus monkey kidney cell cultures.
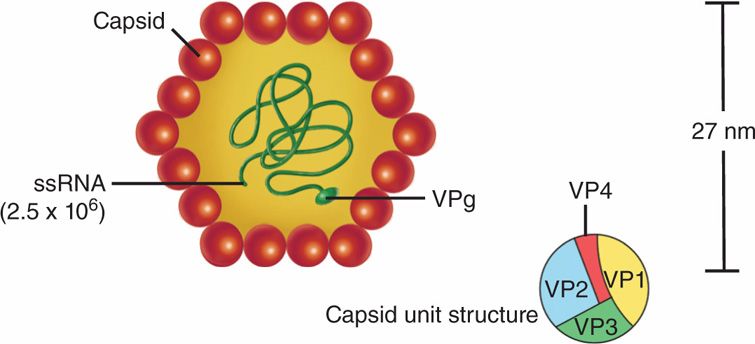
FIGURE 13–1. Diagram of the proposed structure of the hepatitis A virus. The protein capsid is made up of four viral polypeptides (VP1-VP4). Inside the capsid is a single-stranded (ss) molecule of RNA (molecular weight 2.5 × 106), which has a genomic viral protein (VPg) on the 5′ end. (Reprinted with permission of Dr. J. H. Hoofnagle and of Abbot Laboratories, Diagnostic Division, North Chicago, Illinois.)
HAV is a picornavirus with only one serotype
HAV replicates in the cytoplasm, like other positive-sense RNA viruses (Figure 12–1). HAV interacts with the receptor (α2-macroglobulin) on the target cells (liver cells and few other cell types) and enters via receptor-mediated endocytosis (viropexis). The positive-sense RNA is translated into a polyprotein, which is cleaved into various mature proteins, including RNA-dependent RNA polymerase. RNA-dependent RNA polymerase directs transcription of mRNAs to produce viral proteins as well as replication to make full-length viral genomes. The assembly of the progeny viruses takes place in the cytoplasm after the packaging of viral genomes into HAV capsid proteins. Virions are released upon cell lysis.
HAV replicates in the cytoplasm
![]() HEPATITIS A DISEASE
HEPATITIS A DISEASE
EPIDEMIOLOGY
Humans appear to be the major natural hosts of HAV. Several other primates (including chimpanzees and marmosets) are susceptible to experimental infection, and natural infections of these animals may occur. The major mode of spread of HAV is person to person by fecal–oral exposure. Transmission through blood transfusion, though possible, is not an important means of spread, but persons with hemophilia who are given plasma products are at risk. High risk of infection is also observed in men who have sex with men, in illicit drug users, and in travelers from the developed countries visiting developing areas of the world. Although most cases of hepatitis A are not linked to a single contaminated source and occur sporadically, outbreaks have been described. The disease is common under conditions of crowding, and it occurs very frequently in nursing home settings and day care centers. A chronic carrier state has not been observed with hepatitis A; perpetuation of the virus in nature presumably depends on sporadic subclinical infections and person-to-person transmission. Outbreaks of hepatitis A have been linked to the ingestion of undercooked seafood, usually shellfish from waters contaminated with human feces. Common-source outbreaks related to other foods, including vegetables as well as contaminated drinking water, have also been reported.
Fecal–oral transmission
Outbreaks linked to ingestion of uncooked seafood and contaminated food, produce, and water
No chronic carriage
Less than 50% of the general population of the United States now has serologic evidence of HAV infection, and rates have been decreasing since 1970, apparently because of better sanitation, less crowding, and the use of hepatitis A vaccine. In contrast, more than 90% of the adult population in many developing countries shows evidence of previous hepatitis A infection. The risk of clinically evident disease is much higher in infected adults than in children. Patients are most contagious in the 1 to 2 weeks before onset of clinical disease.
More than 90% of adult population is seropositive in developing countries
Subclinical infection is common in children
PATHOGENESIS
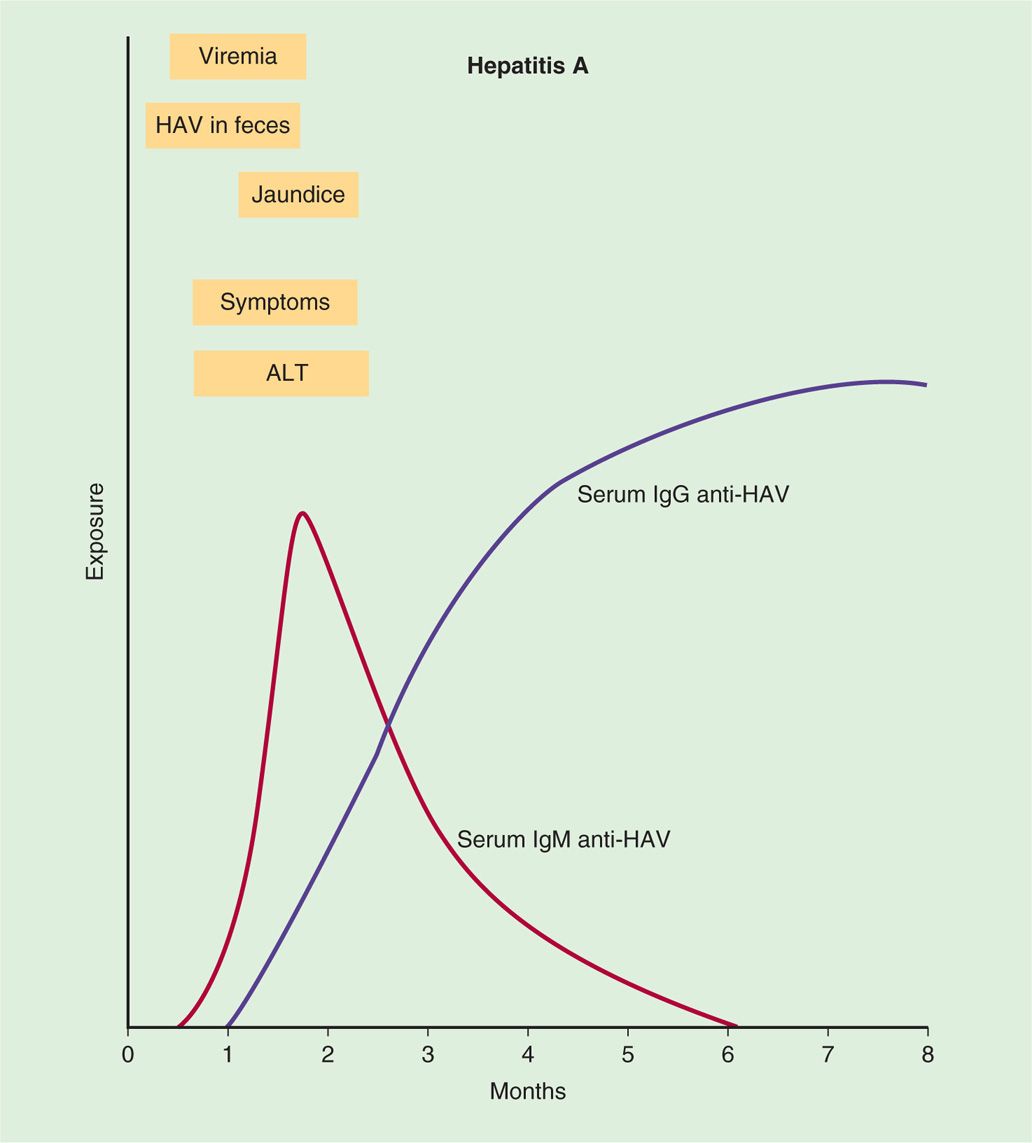
HAV is believed to replicate initially in the enteric mucosa. It can be demonstrated in feces by electron microscopy for 10 to 14 days before onset of disease. In most patients with symptoms of the disease, virus is no longer found in fecal specimens. Multiplication in the intestines is followed by a period of viremia with spread to the liver. The response to replication in the liver consists of lymphoid cell infiltration, necrosis of liver parenchymal cells, and proliferation of Kupffer cells (Figure 13–2). A variable degree of biliary stasis may be present. It is also believed that cytotoxic T lymphocytes (CTLs) damage the hepatocytes. Except in the rare instance of acute hepatic necrosis, the infection is cleared, liver damage is reversed, and HAV does not establish a chronic infection. Initial immune response is the development of HAV-specific IgM antibody followed by appearance of IgG after a few weeks. Detectable levels of IgG antibody to HAV persist indefinitely in serum, and patients with anti-HAV antibodies are immune to reinfection. Although virus-specific IgA has been demonstrated in stool, secretory immunity has not been shown to be important for hepatitis A. The immunopathogenic events associated with HAV infection are shown in Figure 13–3.
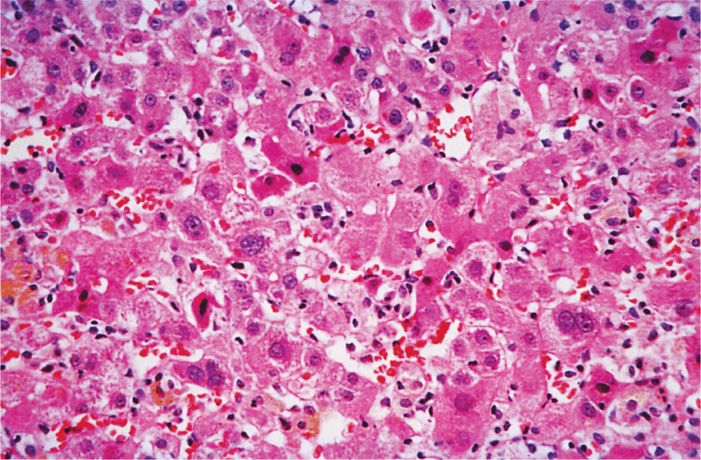
FIGURE 13–2. Acute viral hepatitis, moderately severe. There is a lobular disarray with degeneration, apoptosis, and necrosis of liver cells. Disruption of liver cell plates, hypertrophy of Kupffer cells, a predominantly lymphocytic inflammatory infiltrate, and regeneration of surviving liver cells also are seen. (Reproduced with permission from Connor DH, Chandler FW, Schwartz DQ, et al: Pathology of Infectious Diseases. Stamford CT: Appleton & Lange, 1997.)
FIGURE 13–3. Sequence of appearance of viremia, virus in feces, alanine aminotransferase (ALT), symptoms, jaundice, and IgM and IgG antibodies in hepatitis A virus (HAV) infection.
Contagion is greatest 10 to 14 days before the symptoms appear
IgG-specific antibody is protective
 CLINICAL ASPECTS
CLINICAL ASPECTS
MANIFESTATIONS
In HAV infection, an incubation period of 15 to 45 days (mean 25 days) is usually followed by fever; anorexia (poor appetite); nausea; pain in the right upper abdominal quadrant; and, within several days, jaundice. Dark urine and clay-colored stools may be noticed by the patient 1 to 5 days before the onset of clinical jaundice. The liver is enlarged and tender, and serum aminotransferase and bilirubin levels are elevated as a result of hepatic inflammation and damage. Recovery occurs in days to weeks.
Fever, anorexia, and jaundice are common
Many persons who have serologic evidence of acute HAV infection are asymptomatic or only mildly ill, without jaundice (anicteric hepatitis A). The infection-to-disease ratio is dependent on age; it may be as high as 20:1 in children and approximately 1:1 in older adults. Almost all cases (99%) of HAV are self-limiting. Chronic hepatitis such as that seen with hepatitis B is very rare. In rare cases, fulminant fatal hepatitis associated with extensive liver necrosis may occur (~0.1%).
Chronic infection does not occur
DIAGNOSIS
Antibody to HAV can be detected during early illness, and most patients with symptoms or signs of acute HAV already have detectable antibody in serum. Early antibody responses are predominantly IgM, which can be detected for several weeks and up to several months (Figure 13–3). During convalescence, antibody of the IgG class predominates. The best method for documentation of acute HAV infection is the demonstration of high titers of virus-specific IgM antibody in serum drawn during the acute phase of illness. Because IgG antibody persists indefinitely, its demonstration in a single serum sample is not indicative of recent infection; a rise in titer between acute and convalescent sera must be documented. Immunoelectron microscopic identification of the virus in fecal specimens and isolation of the virus in cell cultures remain research tools.
IgM-specific antibody denotes acute infection
TREATMENT AND PREVENTION
There is no specific treatment for patients with acute hepatitis A. Supportive measures include adequate nutrition and rest. Avoidance of exposure to contaminated food or water or infected persons are important measures to reduce the risk of hepatitis A infection.
 Passive Immunization
Passive Immunization
Passive (ie, antibody) prophylaxis for hepatitis A has been available for many years. Immune serum globulin (ISG), manufactured from pools of plasma from large segments of the general population, is protective if given before or during the incubation period of the disease. It has been shown to be about 80% to 90% effective in preventing clinically apparent type A hepatitis. In some cases, infection occurs but disease is ameliorated; that is, patients develop anicteric, usually asymptomatic, hepatitis A. At present, ISG should be administered to household and intimate contacts of hepatitis A patients and those known to have eaten uncooked foods prepared or handled by an infected person. When clinical symptoms have appeared, the patient is already producing antibody, and administration of ISG is not indicated.
ISG provides temporary protection
 Active Immunization
Active Immunization
Formalin-killed HAV, which is grown in human cell culture, is used as a vaccine that induces antibody titers similar to those of wild-type virus infection, is almost 100% protective, and is now recommended for all children at age 1 and for adults with a high risk of infection. Two doses are given 6 to 12 months apart to achieve long-term protection. In the United States two inactivated HAV vaccines, HAVRIX (GlaxoSmithKline) and VAQTA (Merck & Co) are currently licensed. In addition, a combination vaccine, TWIN-RIX (GlaxoSmithKline) that contains both HAV and HBV antigens given in three or four doses to adults of age 18 years and above, is also available. Based on scientific evidence that active immunization is as effective as ISG if given shortly after exposure, the guidelines were revised in 2007 in the United States to give hepatitis A vaccine after exposure to prevent infection in healthy individuals of 1 to 40 years of age. More importantly, the rates of HAV infection have declined by 92% in the United States since the vaccine was made available in 1995.
Inactivated virus vaccine confers long-term protection
Hepatitis A vaccine prevents postexposure infection
HEPATITIS B
 VIROLOGY
VIROLOGY
STRUCTURE
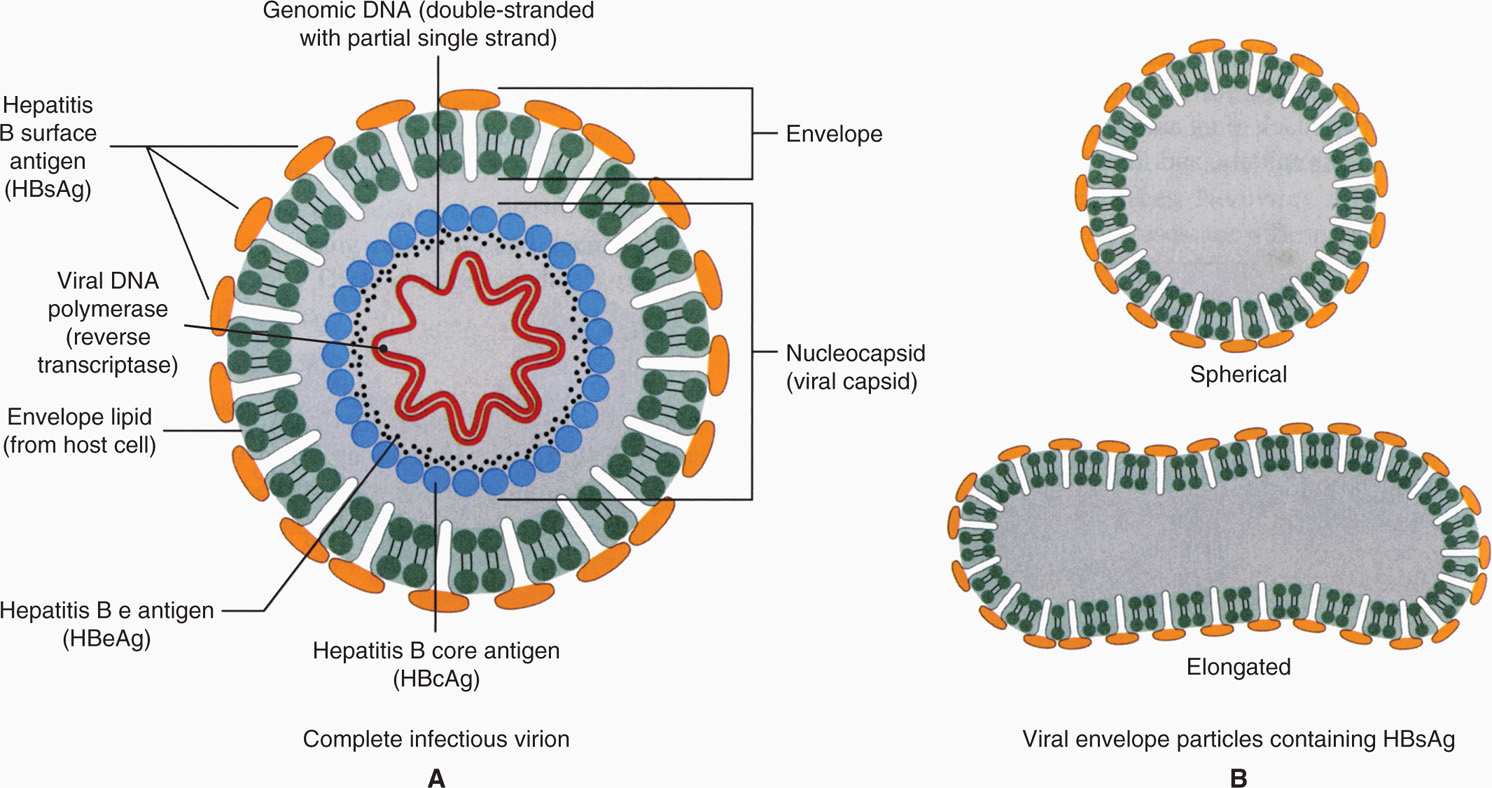
Hepatitis B virus (HBV) is an enveloped DNA virus belonging to the family Hepadnaviridae (hepadnaviruses). It is unrelated to any other human virus; however, related hepatotropic agents have been identified in woodchucks, ground squirrels, and kangaroos. A schematic of the HBV is illustrated in Figure 13–4. The complete virion is a 42 nm spherical particle that consists of an envelope around a 27 nm core. The core comprises a nucleocapsid that contains the DNA genome.
FIGURE 13–4. Schematic diagram of hepatitis B virion. A. The 42 nm particle is the “Dane particle” or the hepatitis B virus. B. The 22 nm particles are the filamentous and circular forms of hepatitis B surface antigen (HbsAg) or protein coat. (Reproduced with permission from Nester EW: Microbiology: A Human Perspective, 6th edition. 2009.)
Smallest known human DNA virus with respect to genome size
The viral genome consists of partially double-stranded DNA with a short, single-stranded piece. It comprises 3200 nucleotides, making it the smallest known DNA virus with respect to genome size but capable of encoding surface (envelope) protein (hepatitis B surface antigen [HBsAg]), core (nucleocapsid) protein (hepatitis B core antigen [HBcAg]), DNA polymerase (reverse transcriptase), and HBx protein (a transcriptional activator). Closely associated with the viral DNA is a viral DNA polymerase, which has RNA-dependent DNA polymerase, DNA-dependent DNA polymerase, and RNase H activities (reverse transcriptase). Another component of the core is hepatitis B e antigen (HBeAg), which is a low–molecular-weight glycoprotein secreted from the infected cells. The virion has a lipid bilayer envelope containing the HBsAg, which is composed of one major and two other proteins. The complete virus particle is called a Dane particle.
Enveloped DNA virus with viral DNA polymerase (reverse transcriptase) activity
Aggregates of HBsAg are often found in great abundance in serum during infection. They may assume spherical or filamentous shapes with a mean diameter of 22 nm (Figure 13–4). HBV DNA can also be detected in serum and is an indication that infectious virions are present. In infected liver tissue, evidence of HBcAg, HBeAg, and hepatitis B DNA is found in the nuclei of infected hepatocytes, whereas HBsAg is found in cytoplasm.
HBsAg is produced in great abundance
There are four major serotypes of HBV (adr, adw, ayr, ayw) based on HBsAg antigenic epitopes. Furthermore, there are eight hepatitis B genotypes (A-H) based on nucleotide sequence variation of HBV genome, which may be associated with different clinical outcomes. These genotypes vary in geographic distribution with genotype A primarily found in North America, Northern Europe, India, and Africa; genotypes B and C in Asia; genotype D in Southern Europe, Middle East, and India; genotype E in West and South Africa; genotype F in South and Central America; genotype G in the United States and Europe, and genotype H in Central America and California.
Found in cytoplasm of infected hepatocytes
REPLICATION CYCLE
The replication of HBV involves a reverse transcription step, and, as such, is unique among DNA viruses (Figure 13–5). HBV has a specific tropism for the liver. However, the receptor for HBV and the mechanism of viral entry are not known. The attachment or adsorption of HBV to hepatocytes (liver cells) is mediated by the envelope protein (HBsAg) of the virus, probably by binding of HBsAg with polymerized human serum albumin or other serum proteins. After viral entry, the partially double-stranded DNA (incomplete) is transported to the nucleus. The double-stranded DNA is organized as two strands. One, a short strand, is associated with the viral DNA polymerase and is of positive polarity.
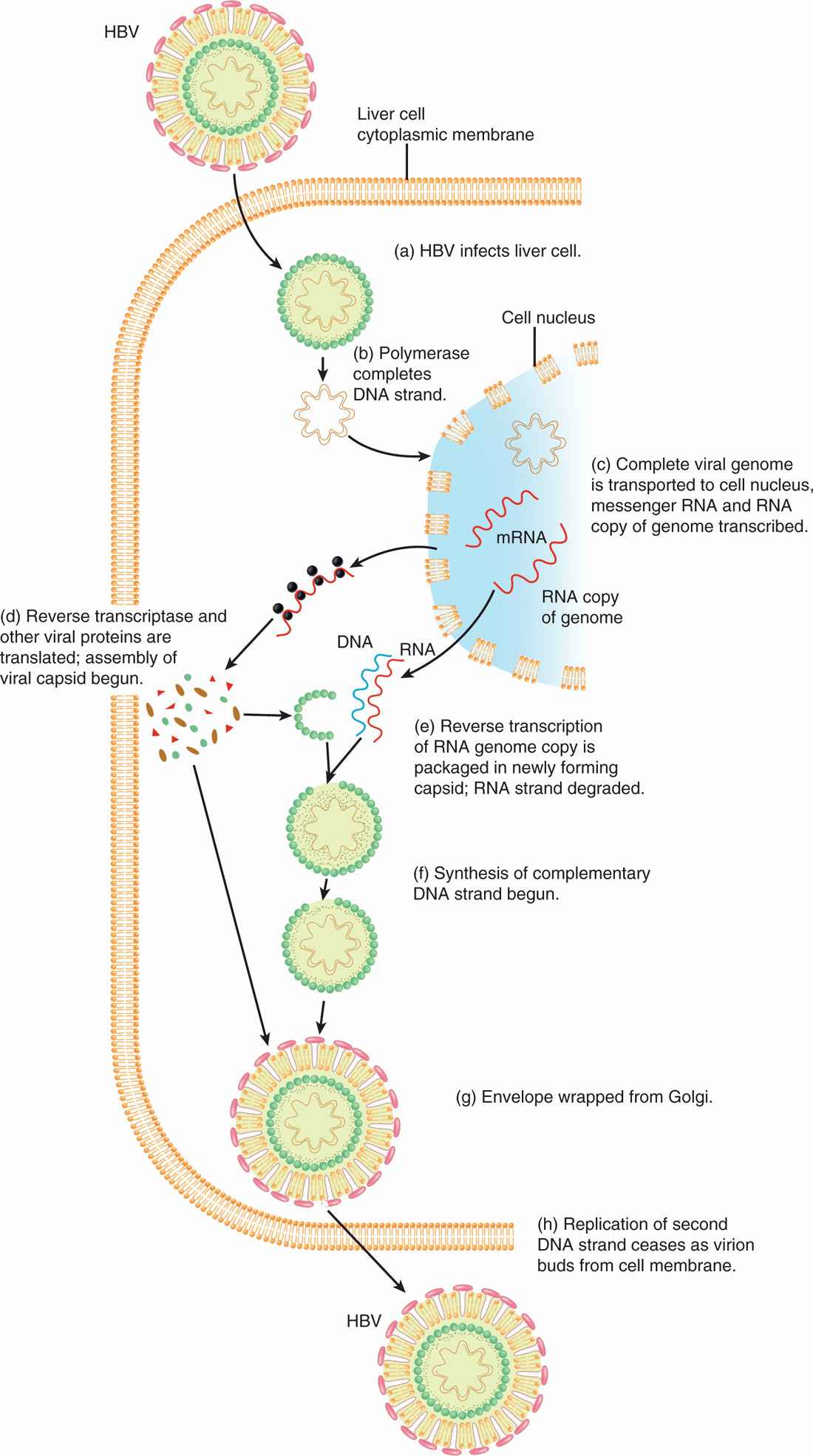
FIGURE 13–5. Replication cycle of hepatitis B virus (HBV). HBV replication requires reverse transcription step, unique among DNA viruses. (Reproduced with permission from Nester EW: Microbiology: A Human Perspective, 6th edition. 2009.)
Partially incomplete double-stranded DNA is formed into a complete double-stranded DNA before transcription
The complete or long strand is complementary and thus is of negative polarity. The partially incomplete strand is formed into a complete double-stranded, circular DNA, which is essential before the transcription can take place. Host RNA polymerase directs the transcription of viral mRNAs to encode early proteins, including HBcAg, HBeAg, and viral DNA polymerase as well as full-length RNA (pregenomic RNA). HBsAg is encoded later and associates with the membranes of endoplasmic reticulum or Golgi apparatus. HBcAg forms the core by enclosing the full-length, positive-sense viral pregenomic RNA along with viral DNA polymerase into maturing core particles late in the replication cycle. These full-length RNA strands form a template for a reverse transcription step in which negative-stranded DNA is synthesized. The RNA template strands are then degraded by ribonuclease H activity. A positive-stranded DNA is then synthesized, although this is not completed before virus maturation in which HBsAg-containing membranes of the endoplasmic reticulum or Golgi apparatus are wrapped over the nucleocapsid core, resulting in the variable-length, short, positive DNA strands found in the virions. The virions are released by exocytosis.
Host RNA polymerase directs synthesis of viral mRNA
Unique replication using a reverse transcriptase step among DNA viruses
Full-length, pregenomic RNA converted to partially incomplete double-stranded DNA by viral DNA polymerase (reverse transcriptase)
Envelope membrane containing HBsAg wrapping from endoplasmic reticulum or Golgi apparatus
HBV DNA has also been found to integrate into the host chromosomes, especially in HBV-infected patients with hepatocellular carcinoma (HCC). However, the significance of integrated HBV DNA in viral replication is not known. Despite extensive attempts, HBV has not been successfully propagated in the laboratory. Humans appear to be the major host; however, as with hepatitis A, infection of subhuman primates has been accomplished experimentally.
Viral DNA integration occurs in some patients with HCC but not essential for viral replication
Humans are the major hosts
![]() HEPATITIS B DISEASE
HEPATITIS B DISEASE
EPIDEMIOLOGY
Hepatitis B infection is found worldwide, with prevalence rates varying markedly among countries, but a total of approximately 400 million persons (Figure 13–6). Chronic carriers constitute the main reservoir of infection: in some countries, particularly in the Far East, up to 5% to 15% of all persons carry the virus, and most are asymptomatic. About 10% of patients with HIV infection are chronic carriers of HBV.
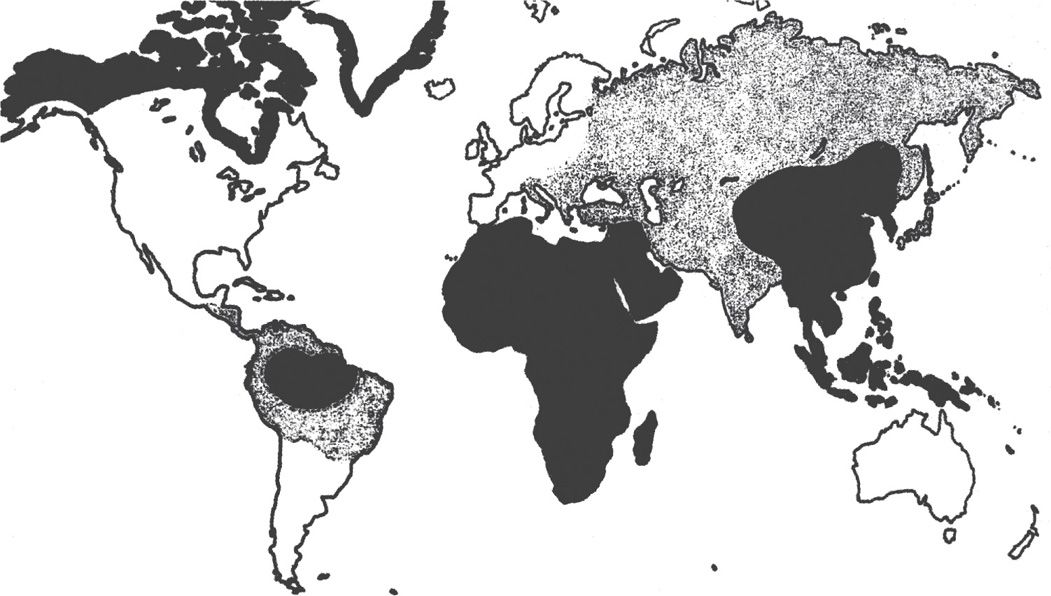
FIGURE 13–6. Worldwide distribution of hepatitis B infection. Areas with high prevalence (>8% of population) are in black, and areas with moderate prevalence (2-7%) are in gray. (Reproduced with permission from Connor DH, Chandler FW, Schwartz DQ, et al: Pathology of Infectious Diseases. Stamford CT: Appleton & Lange, 1997.)
Chronic carriers are common in the Far East
In the United States, an estimated 1.25 million people are infected with hepatitis B, and 300 000 new cases occur annually. About 300 of these patients die of acute fulminant hepatitis, and 5% to 10% of infected patients become chronic HBV carriers. As many as 4000 people die yearly of hepatitis B-related cirrhosis, and 1000 die of HCC. The virus is spread vertically, parenterally, and by sexual contact. Approximately 50% of infections in the United States are sexually transmitted, and the prevalence of HBsAg in serum is higher in certain populations, such as among men who have sex with men, patients on hemodialysis or immunosuppressive therapy, patients with Down syndrome, and injection drug users. Routine screening of blood donors for HBsAg and antibody to HBcAg (anti-HBcAg) has markedly decreased the incidence of postblood transfusion and postplasma products hepatitis B transmission. Multiple-pool blood products still cause occasional cases. Exposure to hepatitis viruses from direct contact with blood or other body fluids, probably through needlestick injuries, has resulted in a risk of hepatitis B infection in medical personnel. Attack rates are also high in the sexual partners of infected patients.
About 50% of HBV infections in the United States are sexually transmitted
Needlestick transmission is a risk for healthcare workers
Hepatitis B infection of infants does not appear to be transplacentally transmitted to the fetus in utero, but is acquired during the birth process by the swallowing of infected blood or fluids or through abrasions. The rate of virus acquisition is high (up to 90%) in infants born to mothers who have acute hepatitis B infection or are carrying HBsAg and HBeAg. Most infants do not develop clinical disease; however, infection in the neonatal period is associated with failure to produce antibody to HBsAg and cell-mediated immune responses probably as a result of an immature immune system, which allows chronic carriage to occur in nearly 90% to 100% of the infected neonates/infants.
Vertical transmission usually occurs during birth process
Chronicity extremely high in vertically infected infants
HCC has been strongly associated with persistent carriage of HBV by serologic tests and by detection of viral nucleic acid sequences integrated in tumor cell genomes. In many parts of Africa and Asia, primary liver cancer accounts for 20% to 30% of all types of malignancies, but in North and South America and in Europe, it is only 1% to 2%. The estimated risk of developing the malignancy for persons with chronic HBV is increased to between 10-fold and more than 300-fold in different populations. The risk of HCC further increases in patients with chronic hepatitis B infection and high viral loads.
Strong association between chronic infection and HCC
PATHOGENESIS
In the past, hepatitis B was known as posttransfusion hepatitis or as hepatitis associated with the use of illicit parenteral drugs (serum hepatitis). However, over the last few years it has become clear that the major mode of acquisition is through close personal contact with body fluids of infected individuals. HBsAg has been found in most body fluids, including saliva, semen, and cervical secretions. Under experimental conditions, as little as 0.0001 mL of infectious blood has produced infection. Transmission is therefore possible by vehicles such as inadequately sterilized hypodermic needles and instruments used in tattooing and ear piercing.
Virus found in blood, saliva, and semen
The factors determining the clinical manifestations of acute hepatitis B are largely unknown; however, some appear to involve immunologic responses of the host. The serum sickness-like rash and arthritis that may precede the development of symptoms and jaundice appear to be related to circulating immune complexes that activate the complement system. In addition, accumulation of these immune complexes in the kidney results in renal damage. Antibody to HBsAg is protective and associated with resolution of the disease. Cellular immunity also may be important in the host response because patients with insufficient T-lymphocyte function have a high incidence of chronic infection with HBV. However, CTLs cause damage to liver by destroying infected cells. Antibody to HBcAg, which appears during infection, is present in chronic carriers with persistent hepatitis B virion production and does not appear to be protective.
Immunologic factors contribute to pathogenicity
Serum sickness-like rash and arthritis precede development of symptoms
Antibody to HBsAg is protective in acute hepatitis
Cellular immunity plays an important role in resolution of the disease
Defects in cellular immunity results in high incidence of chronic infection
The morphologic lesions of acute hepatitis B resemble those of other hepatitis viruses. In chronic active hepatitis B, the continued presence of inflammatory foci of infection results in necrosis of hepatocytes, collapse of the reticular framework of the liver, and progressive fibrosis. The increasing fibrosis can result in the syndrome of postnecrotic hepatic cirrhosis (Figure 13–7).
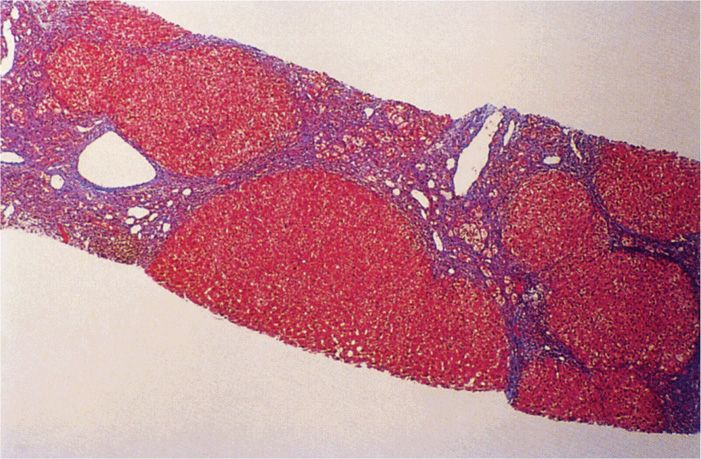
FIGURE 13–7. Cirrhosis of liver in chronic hepatitis B infection (HBV). This is a needle biopsy of Masson trichrome stain that shows cirrhotic nodules and portion of nodules separated by fibrous scars. (Reproduced with permission from Connor DH, Chandler FW, Schwartz DQ, et al: Pathology of Infectious Diseases. Stamford CT: Appleton & Lange, 1997.)
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree